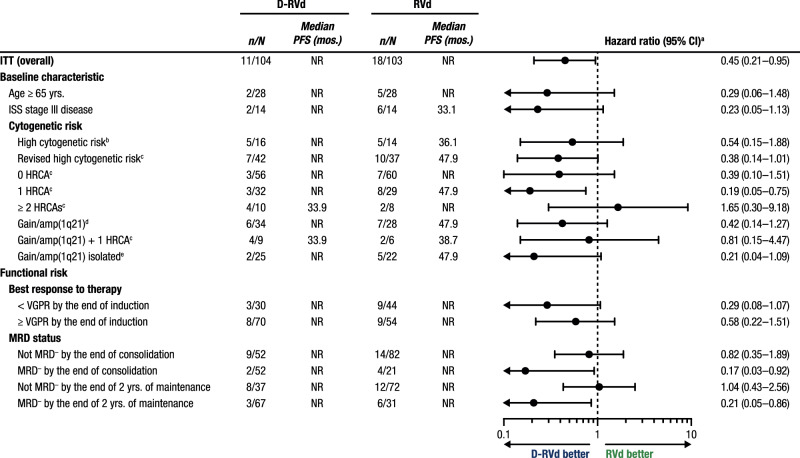
Fig. 4. Subgroup analysis of PFS.
Results of the PFS HR point estimates and their 95% CIs among clinically relevant subgroups of patients. PFS analyses for all groups were evaluated at the time of the final analysis (median overall follow-up, 49.6 months). PFS progression-free survival, D-RVd daratumumab plus lenalidomide/bortezomib/dexamethasone, RVd lenalidomide/bortezomib/dexamethasone, CI confidence interval, ITT intent-to-treat, NR not reached, ISS International Staging System, NE not evaluable, HRCA high-risk cytogenetic abnormality, VGPR very good partial response, MRD minimal residual disease, FISH fluorescence in situ hybridization, HR hazard ratio. aHR and 95% CI are from a Cox proportional hazards model with treatment as the sole explanatory variable. An HR < 1 indicates an advantage for D-RVd. bHigh-risk cytogenetics are defined based on FISH testing as ≥ 1 of the following: del(17p), t(4;14), or t(14;16). cRevised high-risk cytogenetics are defined based on FISH testing as ≥ 1 HRCA: del(17p), t(4;14), t(14;16), t(14;20), or gain/amp(1q21) (≥ 3 copies of chromosome 1q21). dPatients in this group have gain/amp(1q21) with or without other HRCAs (del[17p], t[4;14], t[14;16], or t[14;20]). ePatients with isolated gain/amp(1q21) do not have any other HRCAs.