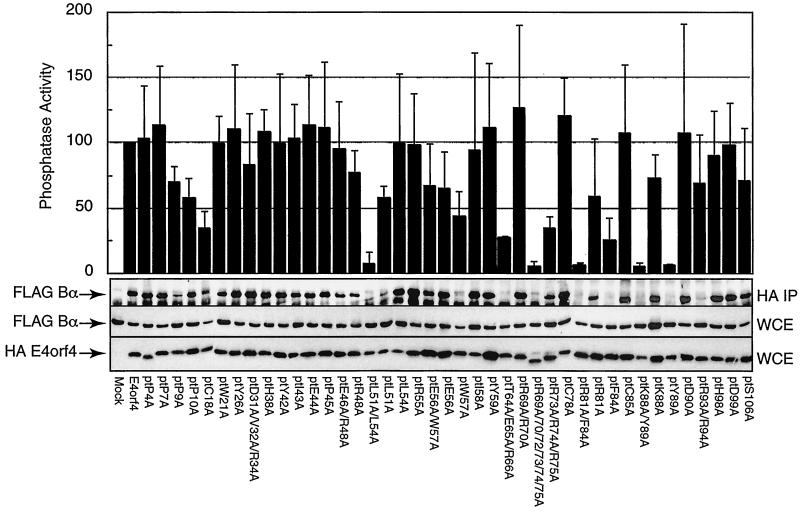
FIG. 5.
Analysis of Bα-subunit binding and associated phosphatase activity of E4orf4 mutants. H1299 cells were lipofected with the HAE4orf4 mutants together with FLAG-tagged Bα. Cell extracts were prepared 24 h postlipofection, and anti-HA immunoprecipitations were performed. Immunoprecipitates were assayed for PP2A activity using a synthetic phosphopeptide as the substrate, as described in Materials and Methods, and results of the average of four separate experiments were plotted relative to those for wild-type E4orf4, which were set at 100% (top). The standard error in these assays was less than 30%. Immunoprecipitates (IP) or whole-cell extracts (WCE) were separated by SDS-PAGE, transferred to nitrocellulose, and then immunoblotted against the FLAG epitope to determine Bα-subunit levels. In addition, blots containing whole-cell extracts were immunoblotted to detect the level of HAE4orf4 by using a murine anti-HA monoclonal antibody. Results from one of four such studies are presented.