Figure 1.
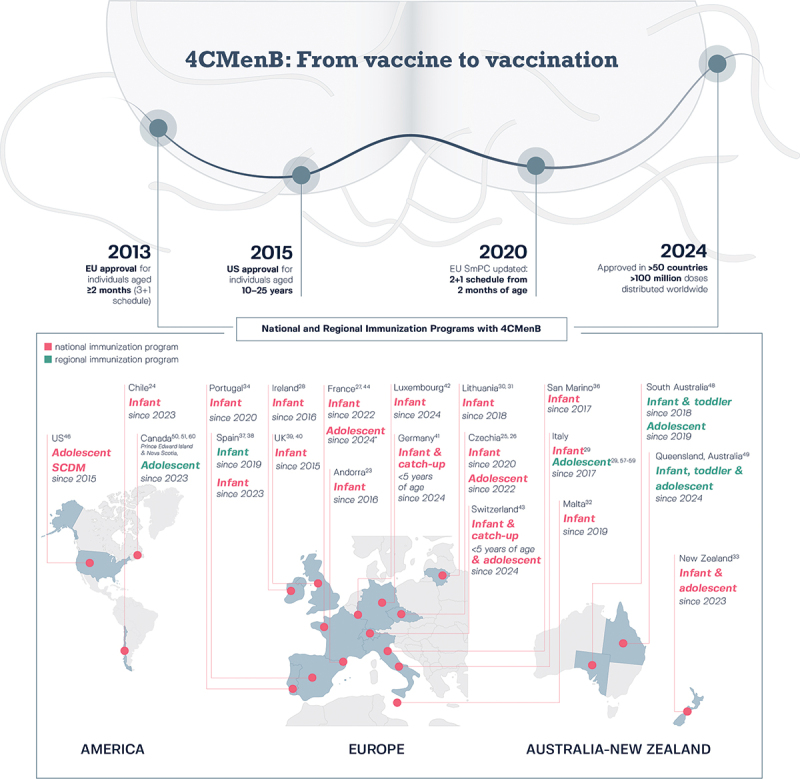
Road map leading to the regulatory approval and distribution of 4CMenB in more than 50 countries worldwide9–11 and to the inclusion of 4CMenB in national and regional immunization programs since initial registration.
*Adolescents/young adults (age 15–24 years) who wish to be vaccinated. 4CMenB, 4-component MenB vaccine; EU, European Union; MenB, meningococcal serogroup B; NIP, national immunization program; RIP, regional immunization program; SCDM, shared clinical decision-making; SmPC, summary of product characteristics; UK, United Kingdom; US, United States.
