Figure 3.
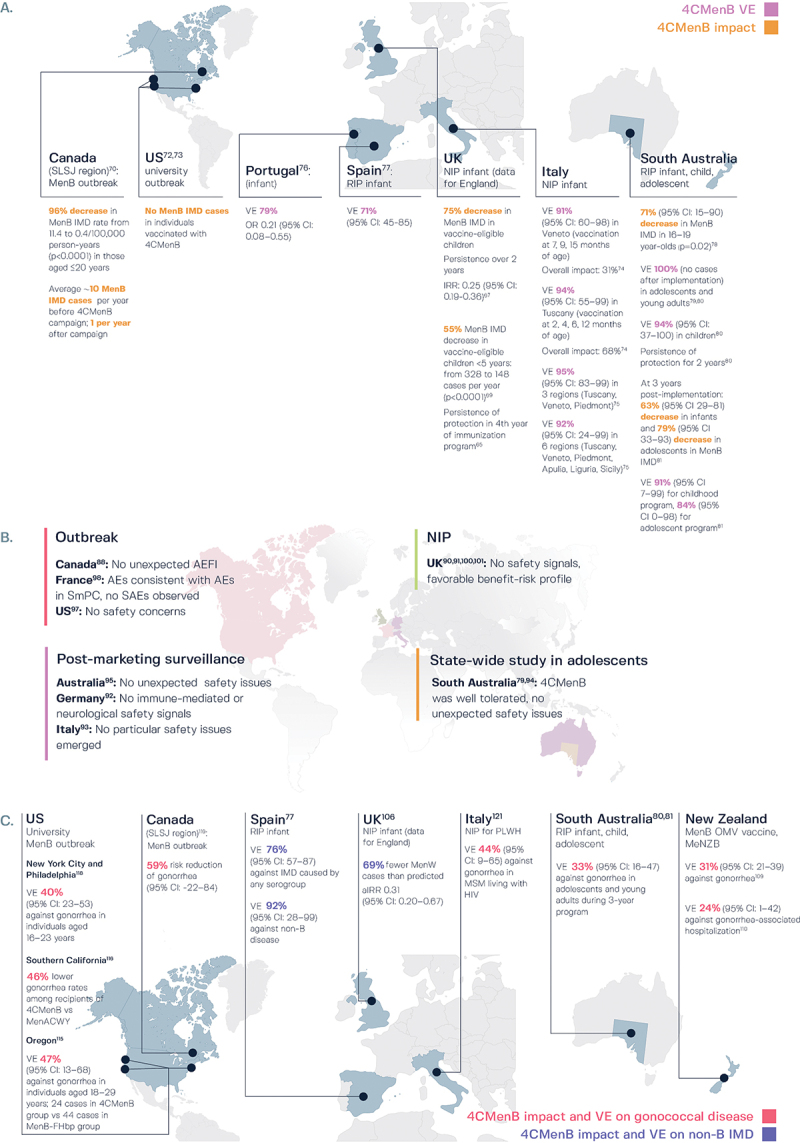
Real-world evidence of the effectiveness, impact, and safety of 4CMenB.
A. Vaccine effectiveness and vaccine impact against invasive meningococcal serogroup B (MenB) disease.
B. Safety profile of 4CMenB.
C. Effectiveness against non-B meningococcal disease and gonococcal disease.
4CMenB, 4-component MenB vaccine; AE, adverse event; AEFI, AE following immunization; aIRR, adjusted IRR; CI, confidence interval; IMD, invasive meningococcal disease; IRR, incidence rate ratio; MenACWY, meningococcal serogroup ACWY vaccine; MenB, meningococcal serogroup B; MenB-FHbp, MenB-factor H binding protein vaccine; MenW, meningococcal serogroup W; MSM, men who have sex with men; NIP, national immunization program; OMV, outer membrane vesicles; OR, odds ratio; PLWH, people living with HIV; RIP, regional immunization program; SAE, serious AE; SLSJ, Saguenay-Lac-Saint-Jean; SmPC, summary of product characteristics; UK, United Kingdom; US, United States; VE, vaccine effectiveness.
