To function correctly, individual myocardial cells rely on normal concentrations of biochemical parameters such as electrolytes, oxygen, hydrogen, glucose, and thyroid hormones, as well as a normal body temperature. Abnormalities of these and other factors affect the electrical activity of each myocardial cell and thus the surface electrocardiogram. Characteristic electrocardiographic changes may provide useful diagnostic clues to the presence of metabolic abnormalities, the prompt recognition of which can be life saving.
It is important to recognise that some electrocardiographic changes are due to conditions other than cardiac disease so that appropriate treatment can be given and unnecessary cardiac investigation avoided
Hyperkalaemia
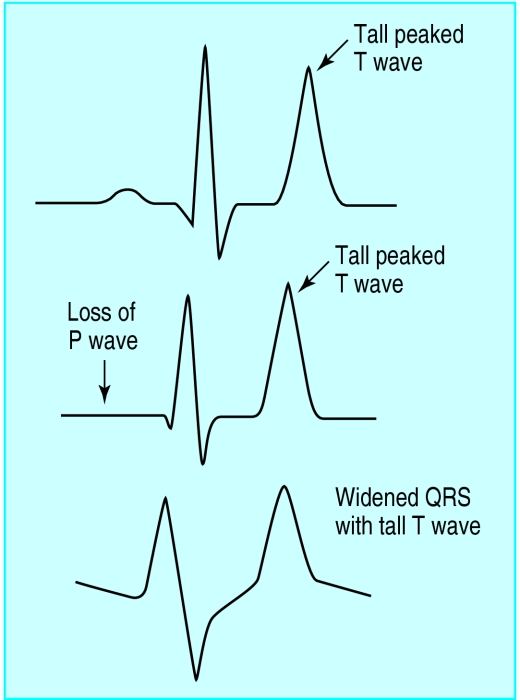
Increases in total body potassium may have dramatic effects on the electrocardiogram. The most common changes associated with hyperkalaemia are tall, peaked T waves, reduced amplitude and eventually loss of the P wave, and marked widening of the QRS complex.
Electrocardiographic features of hyperkalaemia
| Serum potassium (mmol/l) | Major change |
|---|---|
| 5.5-6.5 | Tall peaked T waves |
| 6.5-7.5 | Loss of P waves |
| 7.0-8.0 | Widening of QRS complexes |
| 8.0-10 | Sine wave, ventricular arrhythmias, asystole |
The earliest changes associated with hyperkalaemia are tall T waves, best seen in leads II, III, and V2 to V4. Tall T waves are usually seen when the potassium concentration rises above 5.5-6.5 mmol/l. However, only about one in five hyperkalaemic patients will have the classic tall, symmetrically narrow and peaked T waves; the rest will merely have large amplitude T waves. Hyperkalaemia should always be suspected when the amplitude of the T wave is greater than or equal to that of the R wave in more than one lead.
As the potassium concentration rises above 6.5-7.5 mmol/l, changes are seen in the PR interval and the P wave: the P wave widens and flattens and the PR segment lengthens. As the concentration rises, the P waves may disappear.
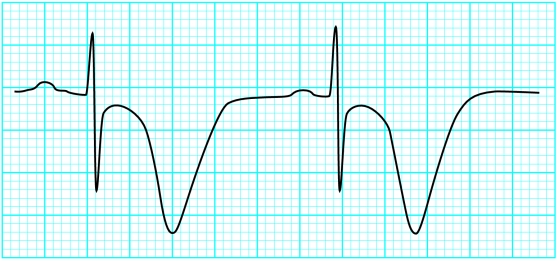
The QRS complex will begin to widen with a potassium concentration of 7.0-8.0 mmol/l. Unlike right or left bundle branch blocks, the QRS widening in hyperkalaemia affects all portions of the QRS complex and not just the terminal forces. As the QRS complex widens it may begin to merge with the T wave and create a pattern resembling a sine wave—a “preterminal” rhythm. Death resulting from hyperkalaemia may be due to asystole, ventricular fibrillation, or a wide pulseless idioventricular rhythm. Hyperkalaemia induced asystole is more likely to be seen in patients who have had chronic, rather than acute, hyperkalaemia.
Hypokalaemia
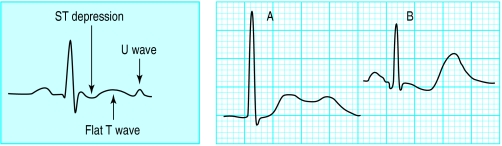
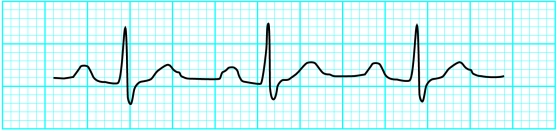
Hypokalaemia may produce several electrocardiographic changes, especially when there is total body depletion of both potassium and magnesium. The commonest changes are decreased T wave amplitude, ST segment depression, and presence of a U wave. Other findings, particularly in the presence of coexistent hypomagnesaemia, include a prolonged QT interval, ventricular extrasystoles, and malignant ventricular arrhythmias such as ventricular tachycardia, torsades de pointes, and ventricular fibrillation. Electrocardiographic changes are not common with mild to moderate hypokalaemia, and it is only when serum concentrations are below 2.7 mmol/l that changes reliably appear.
Electrocardiographic features of hypokalaemia
Broad, flat T waves
ST depression
QT interval prolongation
Ventricular arrhythmias (premature ventricular contractions, torsades de pointes, ventricular tachycardia, ventricular fibrillation)
A prominent U wave in association with a small T wave are considered to be the classic electrocardiographic findings of hypokalaemia. Many authors list a prolonged QT interval as a common finding in hypokalaemia. However, most cases of a presumed prolongation of the QT interval are really QU intervals. Most hypokalaemic patients with true prolongation of the QT interval have coexisting hypomagnesaemia and are at risk of ventricular arrhythmias, including torsades de pointes.
Patients with a potassium concentration below 2.5-3.0 mmol/l often develop ventricular extrasystoles. Hypokalaemia may also be associated with supraventricular arrhythmias, such as paroxysmal atrial tachycardia, multifocal atrial tachycardia, atrial fibrillation, and atrial flutter.
Hypothermia
Hypothermia is present when the core temperature is less than 35°C. As body temperature falls below normal, many cardiovascular and electrophysiological changes occur. The earliest change seen in the electrocardiogram is an artefact due to shivering, although some hypothermic patients have relatively normal traces. The ability to shiver diminishes as body temperature falls, and shivering is uncommon below a core temperature of 32°C.
Electrocardiographic features of hypothermia
Tremor artefact from shivering
Atrial fibrillation with slow ventricular rate
J waves (Osborn waves)
Bradycardias, especially junctional
Prolongation of PR, QRS, and QT intervals
Premature ventricular beats, ventricular tachycardia, or ventricular fibrillation
Asystole
As body temperature falls further, all metabolic and cardiovascular processes slow progressively. Pacemaker (heart rate) and conduction velocity decline, resulting in bradycardia, heart block, and prolongation of the PR, QRS, and QT intervals. At core temperature below 32°C, regular and organised atrial activation disappears and is replaced by varying degrees of slow, irregular, and disorganised activity. If core temperature falls below 28°C, a junctional bradycardia may be seen.
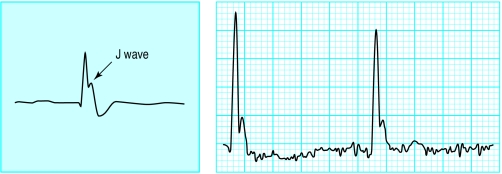
The J wave (Osborn wave) is the most specific electrocardiographic finding in hypothermia. It is considered by many to be pathognomonic for hypothermia, but it may also occasionally be seen in hypercalcaemia and in central nervous system disorders, including massive head injury and subarachnoid haemorrhage.
The J wave may even be a drug effect or, rarely, a normal variant. The J wave is most commonly characterised by a “dome” or “hump” elevation in the terminal portion of the QRS deflection and is best seen in the left chest leads. The size of the J wave often correlates with the severity of hypothermia (<30°C) but the exact aetiology is not known.
Ventricular arrhythmias are the most common mechanism of death in hypothermia. They seem to be more common during rewarming as the body temperature rises through the 28°-32°C range
Thyrotoxicosis
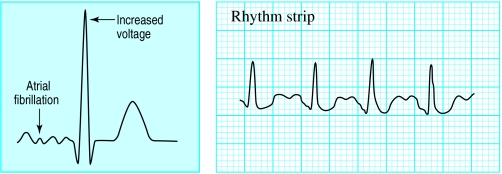
The cardiovascular system is very sensitive to increased levels of circulating thyroid hormones. Increases in cardiac output and heart rate are early features in thyrotoxicosis. The most common electrocardiographic changes seen in thyrotoxicosis are sinus tachycardia, an increased electrical amplitude of all deflections, and atrial fibrillation.
Electrocardiographic features of thyrotoxicosis
Most common findings
Sinus tachycardia
Increased QRS voltages
Atrial fibrillation
Other findings
Supraventricular arrhythmias (premature atrial beats, paroxysmal supraventricular tachycardia, multifocal atrial tachycardia, atrial flutter)
Non-specific ST and T wave changes
Ventricular extrasystoles
About 50% of thyrotoxic patients have a resting pulse rate above 100 beats/min. Atrial tachyarrhythmias are common as the atria are very sensitive to the effects of triiodothyronine. Patients with thyroid storm may develop paroxysmal supraventricular tachycardia with rates exceeding 200 beats/min. Elderly patients may develop ischaemic ST and T wave changes because of their tachycardias. Increased voltage is a common but non-specific electrocardiographic finding in hyperthyroidism, and is more commonly seen in younger patients.
Atrial fibrillation is the most common sustained arrhythmia in thyrotoxicosis, occurring in about 20% of all cases. It is most common in elderly patients, men, those with a particularly high concentration of thyroid hormone, and patients with left atrial enlargement or other intrinsic heart disease. Treatment of atrial fibrillation in thyrotoxicosis is difficult as the rhythm may be refractory to cardioversion. However, most cases revert spontaneously to sinus rhythm when euthyroid. Multifocal atrial tachycardia and atrial flutter with 2:1 conduction, and even 1:1 conduction, may also be seen.
Electrocardiographic features of hypothyroidism
Most common
Sinus bradycardia
Prolonged QT interval
Flat or inverted T waves
Less common
Heart block
Low QRS voltages
Intraventricular conduction defects
Ventricular extrasystoles
Patients with thyrotoxicosis may have other electrocardiographic findings. Non-specific ST and T wave changes are relatively common. Ventricular arrhythmias may be seen, though much less frequently than atrial arrhythmias. Thyrotoxic patients have two or three times the normal number of premature ventricular contractions.
Hypothyroidism
Hypothyroidism causes slowing of the metabolic rate and affects almost all bodily functions, including heart rate and contractility. It causes similar slowing of electrical conduction throughout the heart.
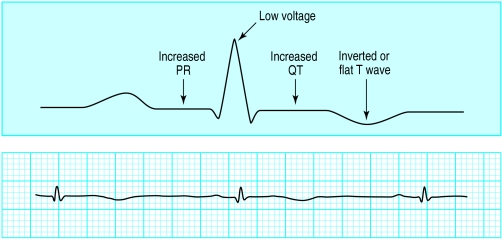
The most common electrocardiographic changes associated with hypothyroidism are sinus bradycardia, a prolonged QT interval, and inverted or flat T waves. Most hypothyroid patients will have a low to normal heart rate (about 50-70 beats/min). Patients with severe hypothyroidism and those with pre-existing heart disease may also develop increasing degrees of heart block or bundle branch block (especially right bundle branch block). Conduction abnormalities due to hypothyroidism resolve with thyroid hormone therapy.
Depolarisation, like all phases of the action potential, is slowed in hypothyroidism, and this results in a prolonged QT interval. Torsades de pointes ventricular tachycardia has been reported in hypothyroid patients and is related to prolongation of the QT interval, hypothyroidism induced electrolyte abnormalities, hypothermia, or hypoventilation.
Hypothyroid patients are very sensitive to the effects of digitalis and are predisposed to all the arrhythmias associated with digitalis intoxication.
Non-specific T wave abnormalities are very common in hypothyroid patients. The T wave may be flattened or inverted in several leads. Unlike with most other causes of T wave abnormalities, in hypothyroidism, associated ST changes are rarely seen
Uncommonly, patients may develop large pericardial effusions, which give rise to electrical alternans (beat to beat variation in QRS voltages). Myxoedema coma should always be suspected in patients with altered mental states who have bradycardia and low voltage QRS complexes (<1 mV) in all leads.
Other non-cardiac conditions
Hypercalcaemia is associated with shortening of the QT interval. At high calcium concentrations the duration of the T wave increases and the QT interval may then become normal. Digoxin may be harmful in hypercalcaemic patients and may result in tachyarrhythmias or bradyarrhythmias. Similarly, intravenous calcium may be dangerous in a patient who has received digitalis. The QT prolongation seen in hypocalcaemia is primarily due to ST prolongation but is not thought to be clinical important.
Hypoglycaemia is a common medical emergency, although it is not often recognised as having electrocardiographic sequelae. The electrocardiographic features include flattening of the T wave and QT prolongation.
Acute electrocardiographic changes commonly accompany severe subarachnoid haemorrhage. Typically these are ST depression or elevation and T wave inversion, although other changes, such as a prolonged QT interval, can also be seen.
Finally, artefacts due to shivering or tremor can obscure electrocardiographic changes or simulate arrhythmias.
Figure.
Serial changes in hyperkalaemia
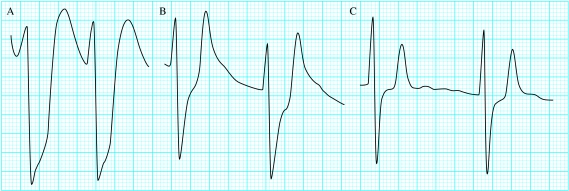
Figure.
Serial changes in patient with renal failure receiving treatment for hyperkalaemia. As potassium concentration drops, the electrocardiogram changes: 9.3 mmol/l, very broad QRS complexes (A); 7.9 mmol/l, wide QRS complexes with peaked T waves and absent P waves (B); 7.2 mmol/l, QRS complex continues to narrow and T waves diminish in size (C)
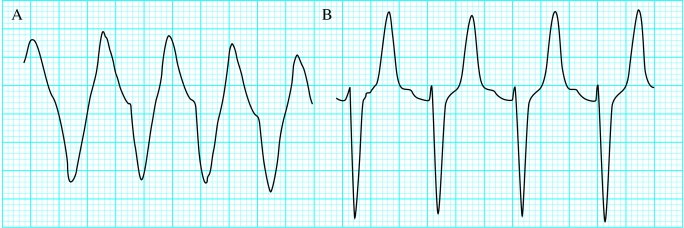
Figure.
Broad complex tachycardia with a potassium concentration of 8.4 mmol/l (A); after treatment, narrower complexes with peaked T waves (B)
Figure.
Left: Diagram of electrocardiographic changes associated with hypokalaemia. Right: Electrocardiogram showing prominent U wave, potassium concentration 2.5 mmol/l (A) and massive U waves with ST depression and flat T waves, potassium concentration 1.6 mmol/l (B)
Figure.
Sinus bradycardia, with a J wave, in a patient with hypothermia—core temperature 29°C (note the shivering artefact)
Figure.
Left: Diagram of electrocardiographic changes associated with thyrotoxicosis. Right: Sinus tachycardia in patient with thyrotoxicosis
Figure.
Top: Diagram of electrocardiographic changes associated with hypothyroidism. Bottom: Bradycardia (note small QRS complexes and inverted T waves) in patient with hypothyroidism
Figure.
Short QT interval in patient with hypocalcaemia (calcium concentration 4 mmol/l)
Figure.
Massive T wave inversion and QT prolongation associated with subarachnoid haemorrhage
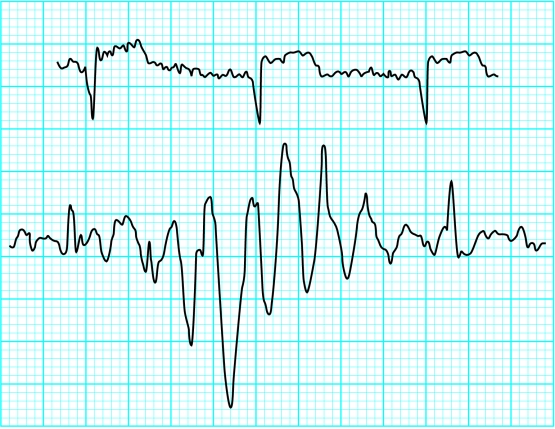
Figure.
Electrocardiographic artefacts—“shivering artefact” in patient with anterior myocardial infarction (top) and electrical interference simulating tachycardia (bottom)
Footnotes
Corey Slovis is professor of emergency medicine and medicine at Vanderbilt University Medical Center in the department of emergency medicine, Nashville, TN, USA. Richard Jenkins is specialist registrar in general medicine and endocrinology at the Northern General Hospital, Sheffield.
The ABC of clinical electrocardiography is edited by Francis Morris, consultant in emergency medicine at the Northern General Hospital, Sheffield; June Edhouse, consultant in emergency medicine, Stepping Hill Hospital, Stockport; William J Brady, associate professor, programme director, and vice chair, department of emergency medicine, University of Virginia, Charlottesville, VA, USA; and John Camm, professor of clinical cardiology, St George's Hospital Medical School, London. The series will be published as a book in the summer.