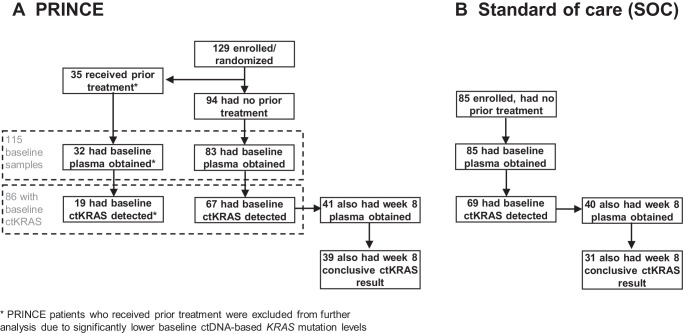
Fig. 1. CONSORT diagram showing the PRINCE clinical trial and standard of care (SOC) validation cohorts.
Plasma samples were obtained from 200 total patients, including 115 PRINCE (A) and 85 SOC (B) patients. Altogether, 281 samples were analyzed, including: 115 PRINCE samples at baseline (32 for patients with prior therapy plus 83 for patients with no prior therapy), 41 PRINCE samples at week 8 (for patients with no prior therapy), 85 SOC samples at baseline, and 40 SOC samples at week 8. All SOC patients were therapy-naive. Among the 67 PRINCE patients with ctKRAS detected in baseline plasma, 41 also had a week 8 plasma obtained but 26 were unavailable due to the following reasons: 15 discontinued treatment prior to week 8, 6 died prior to week 8, 3 received a dose at week 8 but there was no blood drawn, and 2 did not receive a dose at week 8 due to an adverse event. Among 69 SOC patients with ctKRAS detected in baseline plasma, 40 also had a week 8 plasma obtained but 29 were unavailable due to the following reasons: 8 either discontinued treatment or were lost to follow-up, 13 died prior to week 8, 5 had a follow-up blood draw outside of the week 8 window, and 3 did not have week 8 samples available at the time of analysis. Source data are provided as a Source Data file.