Figure 1.
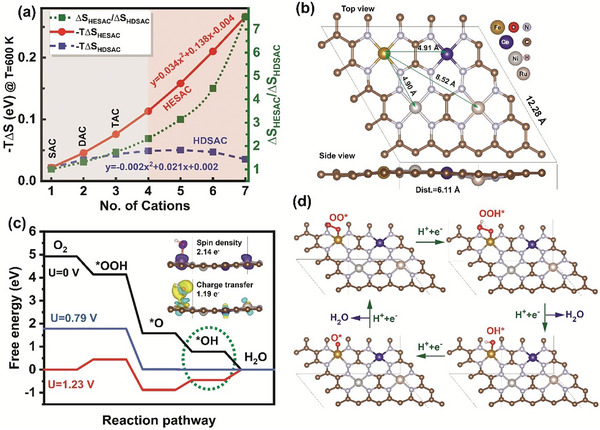
Oxygen reduction reaction (ORR) mechanism on FeCoNiRu‐HESAC. a) Entropy behavior of HDSAC and HESAC versus the number of cations in the 4 × 4 supercell of nitrogen‐doped Graphene. b) Lateral and side views of the DFT‐optimized structure of FeCoNiRu‐HESAC with the averaged intermetallic distance (Dist.) of 6.11 Å. Fe, Co, Ni, and Ru are anchored on the moiety side. c) Free energy change of ORR intermediates catalyzed by FeCoNiRu‐HESAC at the applied potentials of 0.00 V (black), 0.79 V (blue), and 1.23 V (red), indicating that the potential determining step is the desorption of OH* intermediate (shown in a dashed green cycle). The inset shows the spin and charge transfer of FeCoNiRu‐HESAC in the presence of OH* intermediate. Red and blue colors correspond to the beta and alpha spin density, respectively (Isosurface value = 0.02 e Å−3), while yellow and green colors indicate the charge availability and deficiency, respectively (Isosurface value = 0.005 e Å−3). d) Side view of ORR intermediates on Fe active site of FeCoNiRu‐HESAC.
