Figure 4.
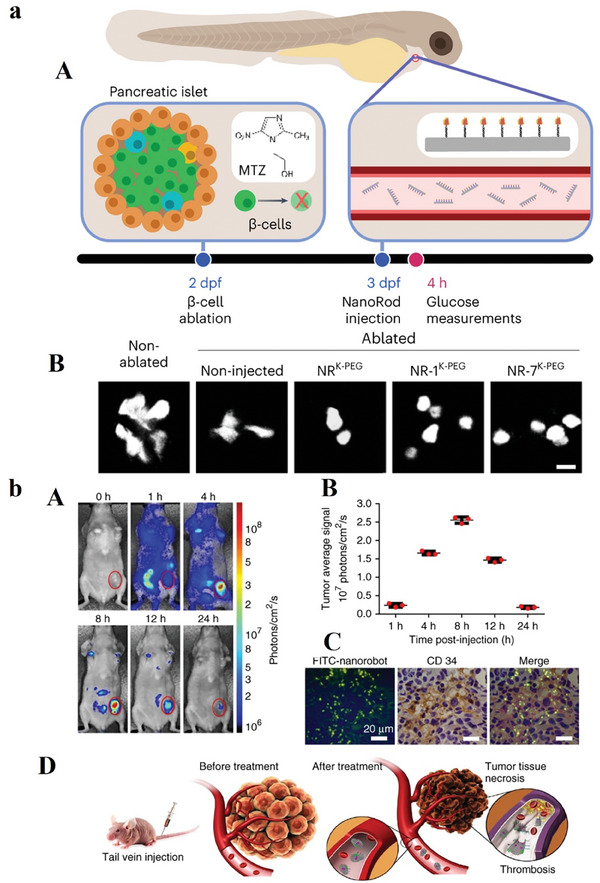
a) (A) The depiction provides the zebrafish model's overview, wherein the enzyme nitro‐reductase (NTR) is expressed under the insulin promoter regulatory control. This regulatory arrangement facilitates the conversion of the MTZ compound to a cytotoxic component, ultimately resulting in the ablation of β‐cells. Larvae underwent MTZ treatment at 2 dpf for a duration of a day. Double‐transgenic larvae, denoted as Tg(ins: CFP‐NTR); Tg(ins: Kaede), were employed for the visualization of β‐cells using the fluorescent protein Kaede. Intravenous injections of NRK‐PEG, NR‐1K‐PEG, or NR‐7K‐PEG were administered at 3 dpf, and the levels of free glucose were subsequently measured at 4 h postinjection. (B) The utilization of confocal microscopy has been employed to capture the expression of the Kaede fluorescent protein within pancreatic β‐cells under the specified experimental conditions. The scale bar corresponds to 10 µm. Reproduced with permission from.[ 70 ] Copyright 2023, Nature Nanotechnology. b) (A) Optical imaging was conducted on a mouse hosting a breast tumor of a human (MDA‐MB‐231) before and after the intravenous introduction of Cy5.5‐labeled nanorobots. A discernible intense fluorescent signal was exclusively detected in the tumor site 8 h postinjection. The 0 h time point designates the pre‐injection state. The presented images demonstrate the 3 experiments. (B) Quantification of in vivo fluorescence intensity of tumor region at designated time intervals following nanorobot administration. Error bars show the mean ± standard deviation of 3 experiments. (C) FITC‐labeled nanorobots intravenous injection into mice with tumors (MDA‐MB‐231). Tumors have been harvested 8 h postinjection, and subsequent staining with an anti‐CD34 antibody enabled examination through confocal microscopy. The nanorobot (green) is observed in regions rich in blood vessels (anti‐CD34; brown). Nuclei are denoted in blue. The presented images show 3 different experiments, with scale bars set at 20 µm. (D) illustration elucidates the nanorobot‐Th therapeutic mechanism within tumor vessels. DNA nanorobot‐Th administration via tail vein injection to breast tumor xenografted mice targets tumor‐associated vessels, delivering thrombin. The nanorobot‐Th, recognizing nucleolin, binds to vascular endothelium, subsequently opening to expose encapsulated thrombin, inducing localized thromboses, tumor infarction, and cell necrosis. Reproduced with permission from.[ 71 ] Copyright 2018, Nature Biotechnology.
