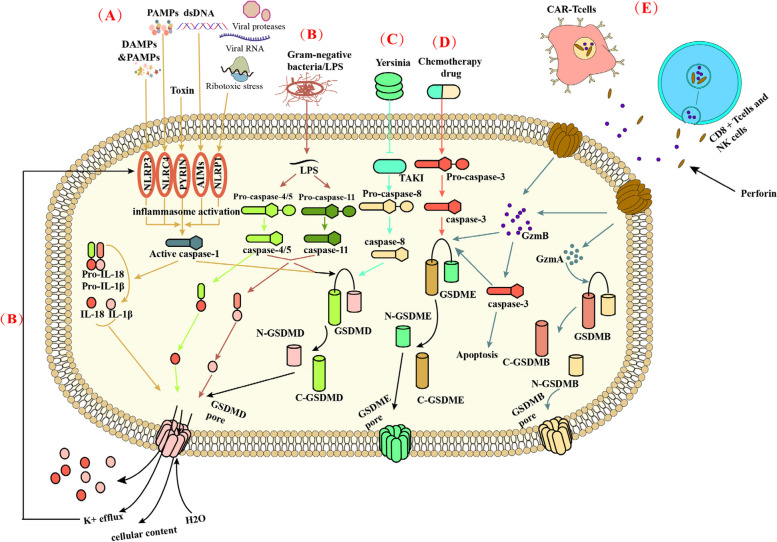
Fig. 2.
Molecular mechanisms of Pyroptosis. A In the canonical pathway, PAMPs and DAMPs Stimulate the corresponding inflammasome, leading to the activation of the inflammasome. Activated inflammasome can lead to the activation of caspase-1. Then, caspase-1 cleaves GSDMD form the N-terminus and C-terminus of the GSDMD. The N-terminus of GSDMD perforates the cell membrane by forming nonselective pores in the cell membrane, thus causing water influx, lysis, and death. At the same time, activated caspase-1 can promote the conversion of pro-IL-1β and pro-IL-18 to IL-1β and IL-18, which are out of the cells from the previously formed pores. B In the noncanonical pathway, cytosolic LPS activates caspase-4/5 and caspase-11, leading to pyroptosis through GSDMD cleavage. GSDMD-NT can also create pores in the cell membrane, causing K + and cellular content efflux, cell swelling, and rupture. This process facilitates the maturation and release of pro-IL-18 and IL-Iβ through K + efflux. Recent studies have revealed that activated human caspase-4/5 can directly cleave and activate IL-18, unlike mouse caspase-11. Additionally, all caspase-4/5/11 can cleave IL-1β to inhibit IL-1β signaling. C At Yersinia infection, the effector protein (Yopj) expressed by Yersinia can promote the conversion of pro-caspase-8 to caspase-8, thus mediating the pyroptosis caused by GSDMD. D Chemotherapy drugs can allow pro-caspase-3 to convert to caspase-3. And then caspase-3 cleaves the GSDME, resulting in pyroptosis. E In the granzyme-mediated pathway, CAR-T cells, CD8+T cells, and NK cells rapidly activate caspase-3 in target cells by releasing GzmB, and then GSDME was activated, causing extensive pyroptosis and apoptosis. At the same time, GzmA released from CD8+T cell and NK cell could promote cleavage of GSDMB into the N and C terminal. The N terminal of GSDMB forms holes in the cell membrane leading to pyroptosis