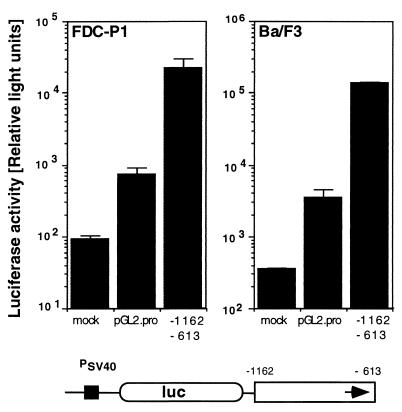
FIG. 2.
Activity of the MMTV LTR enhancer in hematopoietic progenitor cell lines. FDC-P1 and Ba/F3 cells were transfected either in the absence of a reporter gene plasmid (mock) or with the enhancer test plasmid pGL2-Promoter (pGL2-Pro) or pGL2-Pro containing the Mtv-1 LTR fragment from positions −1162 to −613 cloned into the unique BamHI site 3′ of the promoter-reporter gene cassette (−1162 to −613). The schematic structure of the reporter plasmid is shown below the graph. An enhancerless SV40 promoter (PSV40) controls the expression of the luciferase reporter gene (luc). The MMTV enhancer element is represented by an open box, with an arrow indicating the positive orientation of the fragment. The IL-3-dependent mouse progenitor cell lines Ba/F3 (16) and FDC-P1 (5), gifts from U. Klingmüller, were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum, 10% WEHI 3B-conditioned medium, penicillin (100 IU/ml), and streptomycin (100 μg/ml). DNA was introduced by electroporation with a Bio-Rad Genepulser. Equimolar amounts of test plasmids (2 pmol) were linearized outside of the cloned provirus with a restriction enzyme. Carrier plasmid pGEM-2 and TE (10 mM Tris, 1 mM EDTA [pH 7.0]) were added to a constant DNA amount and volume. Cells were harvested after 24 h, washed twice in phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4), and lysed in reporter lysis buffer (Promega). The luciferase assay system (Promega) was used for detection of firefly luciferase (duplicate assays). Light emission was determined for 10 s in a model ILA911 (Tropix) or a model Lumat LB 9501 (Berthold, Wildbad, Germany) luminometer. Protein concentration in cellular extracts was measured by the Bio-Rad protein assay. Results are expressed as relative light units and represent the arithmetic means ± standard deviations from at least three parallel experiments with two different DNA preparations.