FIG 2.
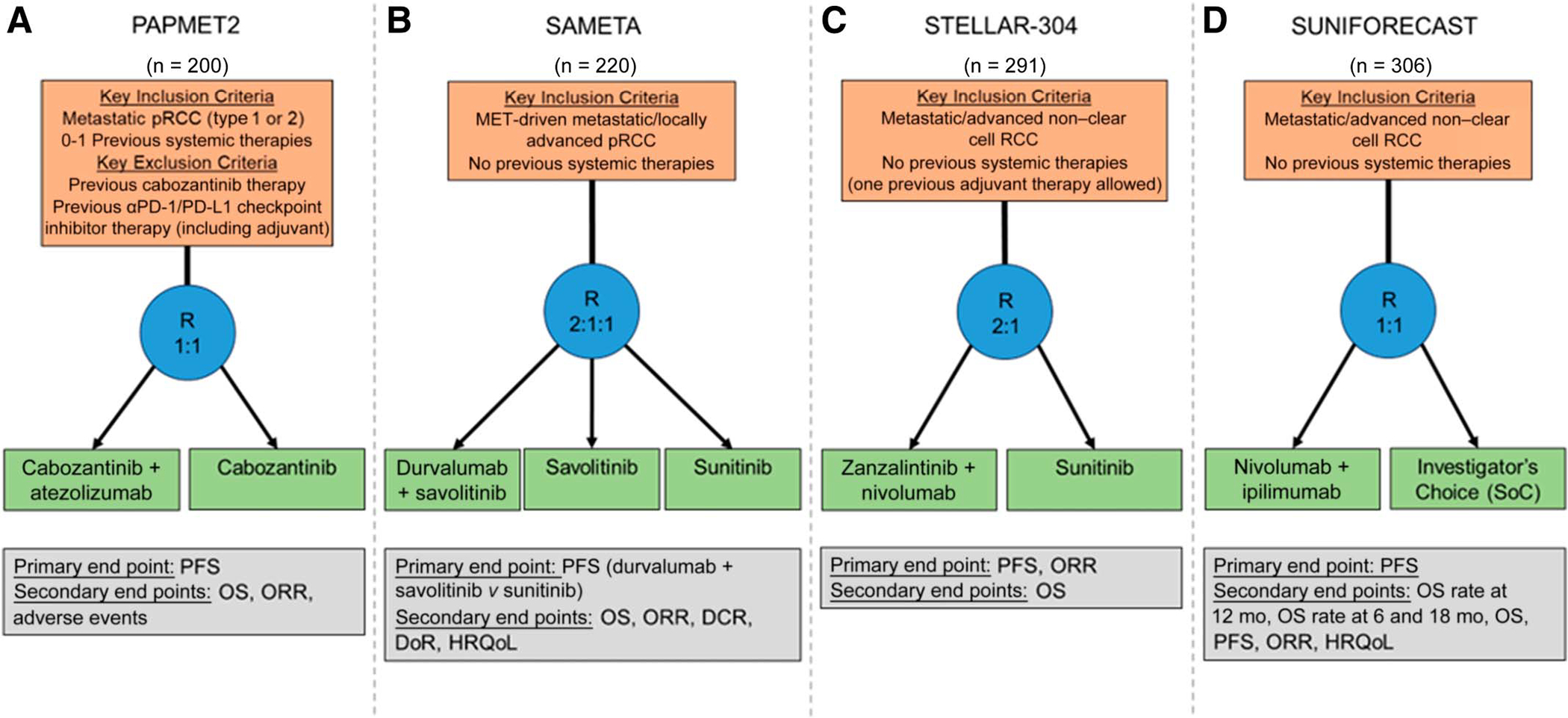
Schematics of ongoing randomized trials for patients with pRCC: (A) PAPMET2 (ClinicalTrials.gov identifier: NCT05411081), (B) SAMETA (ClinicalTrials.gov identifier: NCT05043090), (C) STELLAR-304 (ClinicalTrials.gov identifier: NCT05678673), and (D) SUNIFORECAST (ClinicalTrials.gov identifier: NCT03075423). DCR, disease control rate; DoR, duration of response; HRQoL, health-related quality of life; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; pRCC, papillary renal cell carcinoma; R, randomize; RCC, renal cell carcinoma; SoC, standard of care.
