Abstract
Background and purpose
Female gender, younger age and stressful life events are known predisposing factors for functional neurological disorders (FNDs). Employment in a healthcare profession has also been suggested to be a predisposing factor. We set out to conduct a large‐scale case–control study to estimate the rate employment in a healthcare profession among people with FND.
Methods
We included 200 consecutive patients with a confirmed diagnosis of FND, referred to our clinic at University Hospital Bern Switzerland between October 1, 2016, and August 1, 2019. In addition, we included a control group of 200 patients with a confirmed neurological disorder, matched for age and gender, seen during the same period. The primary endpoint was to compare the prevalence of healthcare professionals between the groups. We also describe the clinical manifestations and concomitant psychiatric diagnoses in the FND cohort.
Results
Female gender was predominant (70%), and the participants’ mean age was 37 years. The proportion of healthcare professionals in the FND patients was 18% (33/186), which was significantly higher than in the control group, in which it was 10.6% (17/189; p = 0.019, 95% confidence interval odds ratio 1.168–4.074). Most healthcare professionals in both cohorts were nurses (21/33 among FND patients, 10/17 among controls). Among FND patients, 140 (70%) had motor symptoms and 65 (32.5%) had a concomitant psychiatric diagnosis.
Conclusion
This case–control study confirmed a higher rate of employment in healthcare professions in patients with FND, suggesting two potential mechanisms of FND: exposure to models/specific knowledge about neurological symptoms or stress‐related professional factors. This warrants further studies on underlying mechanisms and prevention.
Keywords: disease modeling, exposure, functional neurological disorder, healthcare profession
INTRODUCTION
Functional neurological disorders (FNDs) are characterized by the presence of a broad range of neurological symptoms (weakness, abnormal movement or sensations, dizziness) not explained by classical neurological conditions (such as stroke, Parkinson's disease, and multiple sclerosis) but for which specific brain dysfunctions have been found [1]. FNDs are amongst the most frequent diagnoses in neurology services [2, 3, 4, 5], as well as in neurology reviews for patients admitted in non‐neurology care settings [6]. Typically, patients experience a chronic course with socioeconomic consequences [7, 8, 9], often undergoing several diagnostic procedures [10] or facing misdiagnosis before being diagnosed with FND [11]. A growing interest in this topic has led to an increase in research trying to characterize, if not causal agents, at least risk factors, such as stressful life events, experienced neglect, comorbid neurological or psychiatric conditions and pain, as well as (epi)genetic factors [3, 5, 11, 12, 13, 14]. Other potential risk factors such as exposure to and modeling of disease were discussed but not yet fully understood [15, 16, 17, 18, 19, 20]. It has been hypothesized that having been exposed to the observation of neurological symptoms, within the family or during professional experience, may represent a risk factor, serving as a “model” of how a physical symptom can express itself and favoring the emergence of similar symptoms [16, 19, 20, 21]. This modeling would be different from a conscious imitation or even from feigning a symptom. Having a model might change predictions of how the brain is supposed to sense and act on the environment, according to a Bayesian framework of FND [22, 23]. Even without evidence of genetic transmission, familial cases with same‐symptom presentation as their affected family member (e.g., tremor in grandmother and granddaughter) occur [18]. A recent outbreak of functional tics has been linked to exposure to social media where patients with tics were showing and explaining their symptom [24, 25]. This might represent a form of exposure and thus one risk factor—among others—for developing functional tics.
Studying family or social media exposure requires extended history and is prone to recall bias. Studying exposure to neurological patients can be done by recording an individual's professional occupation. Indeed, being a member of the healthcare profession as a risk factor has been discussed in several publications, which report a high frequency (as high as 45%) of this [21], and has even been suggested as a diagnostic criterion [4, 19, 20]. However, controlled studies yielded mixed results: significantly more healthcare workers were found in a cohort of 322 FND patients (19%) compared to 644 psychiatric controls (8%) [26] but no statistically significant differences were found when studied in small samples and in subgroups, such as dystonia (25% in 132 functional dystonia vs. 20% in 148 focal dystonia) [27] and tremor (33.3% in 12 functional tremor vs. 12.1% in 33 essential tremor) [28].
To verify if working in healthcare and being potentially exposed to neurological symptoms can represent a risk factor for FND, we set out to systematically study the frequency of healthcare professionals in mixed FND patients by means of a large case–control study design.
METHODS
We conducted a single‐center, case–control study based on electronic data collection and chart reviews from patients referred to the outpatient clinic of the Neurology Department of the University Hospital, Inselspital, Bern, Switzerland. All included patients signed a general informed consent or were informed about the use of clinical data for research purposes. The study protocol was reviewed and accepted by the local Ethics Committee of Canton Bern, Switzerland (BASEC: 2021‐01908).
The Neurology Department of Bern is a tertiary academic hospital, receiving referral from a region (Kanton Bern) representing a catchment area of 1 million inhabitants, as well as referrals throughout the country. Referrals are triaged into specific clinics of the outpatient department by a trained neurologist based on detailed written information from the referring physician.
Cases: FND clinic patients
All consecutive patients referred to the FND Clinic between October 1, 2016 and August 1, 2019 were screened. Included were patients with confirmed FND diagnosis according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) criteria (F44.4‐7). Diagnosis was assessed by fellows or attending doctors of the FND Clinic of the University Hospital, Inselspital, Bern, Switzerland. As the clinic was newly opened on October 1, 2016, this cohort of patients represents all new referrals.
Controls: general neurology patients
Patients referred to the General Neurology Clinic between October 1, 2016 and August 1, 2019 were randomly selected to match the cases for age and gender. To match age, the cases were divided into subgroups as follows: <20 years; 20–65 years, grouped in 5‐year ranges (i.e., 20–24.99 years, 25–29.99 years, etc.); and >65 years. An equal number of gender‐matched controls in each age subgroup was then selected. As the clinic has been running for many years, this cohort could include either new referrals or follow‐up consultations.
Extraction of data
We collected the following data: demographics (age, gender, current occupation, or occupation before retirement, disability allowance), date of first consultation, date of symptom onset and final diagnosis. Detailed clinical features (kind and severity of symptoms, therapy, concomitant neurological or psychiatric diagnosis) were collected only for the FND patient cohort. All data were available in the digital medical records.
Gender
We recorded the gender of patients, as self‐reported at registration in the medical records. Biological sex was not considered.
Age at event
Age at symptom onset was calculated based on birth date and descriptions in the records. When date of symptom onset was available, the precise date (day/month/year) was recorded. When only the month was mentioned, we recorded the date as 01/month/year. When only the year was mentioned, we recorded the date as 01/01/year.
Profession
Swiss law sets standards to classify the population's professional status (retirement age: 65 years for men, 64 years for women). We coded all patients without professional activity above that age as “retired”. All patients aged below those thresholds were considered as “active” (even if unemployed), or as on disability allowance if they were receiving a benefit due to inability to work caused by the disease. All students of any age were coded as “student” independently of educational stage (e.g., primary, secondary, tertiary education).
Healthcare professionals were defined as any workers who were working or had once worked in any activity with regular patient contact and concomitant basic knowledge of human neuropathophysiology (e.g., a psychologist or pharmacist), as well as students of medical professions. Administration workers in the healthcare sector were not considered to be healthcare professionals. Participants without information about their job were excluded from this sub‐analysis.
Power analysis
Assuming an incidence of healthcare workers of approximately 20% among cases and approximately 10% among controls (based on O'Connell) [26], and considering a type I error as α = 0.05, a sample size of 398 subjects (199 cases and 199 controls) would result in a power of 80%. We thus aimed for a sample consisting of 200 cases and 200 controls.
Statistical analysis
Statistical analysis was based on relative and absolute frequencies. Comparison of the cohorts was focused on profession and was tested using the chi‐squared test. Odds ratios (ORs) were used for effect size testing, while the phi‐coefficient was calculated to measure the association strength. We conducted a Shapiro–Wilk normality test to check the distribution of the whole population based on the age at event in both cohorts. The Wilcoxon signed ranked test was applied to establish whether the two cohorts differed significantly regarding age distribution. All statistical analyses were performed using R Core Team software (version R 4.0.3 GUI 1.73) [29].
RESULTS
Patients
We screened a total of 447 patients referred to the FND clinic in the studied period; 109 did not have a confirmed FND diagnosis after first consultation. A total of 138 patients did not give consent for use of their clinical data for research purposes or were not informed. We included 200 cases of confirmed FND. As neurological controls, we randomly selected 200 gender‐ and age‐matched patients from the neurological department. The analyzed cohort consisted of 400 subjects.
Demographics
Among FND cases the large majority were female (140 females vs. 60 males, 70%). The mean (SD) age at symptom onset for FND patients was 37.0 (15.1) years. Given a Shapiro–Wilk normality test of p < 0.001, a nonsignificant Wilcoxon signed rank test (p = 0.77) suggested no difference in mean age and age distribution between the two cohorts. See Table 1 for details.
TABLE 1.
Demographics.
| Category | FND cohort (Total 200) | Control cohort (Total 200) | Statistical significance |
|---|---|---|---|
| Gender | N = 200 | N = 200 | |
| Male, n (%) | 60 (30) | 60 (30) | |
| Female, n (%) | 140 (70) | 140 (70) | |
| Age at symptom onset, years | 37.0 (±15.1) | 37.5 (±15.2) |
Shapiro–Wilk normality test p < 0.001 Wilcox signed rank test p = 0.77 |
| Occupational status | N = 200 | N = 200 | |
| Student, n (%) | 17 (8.5) | 4 (2) | |
| Active, n (%) | 149 (74.5) | 175 (87.5) | |
| Retired, n (%) | 11 (5.5) | 9 (4.5) | |
| Disability allowance, n (%) | 23 (11.5) | 12 (6) |
p = 0.076, φ = 0.10, OR = 2.035 95% CI OR = 0.983–4.123 |
| Profession | N = 186 | N = 189 | |
| Healthcare, n (%) | 33 (18) | 17 (10.6) |
p = 0.019, φ = 0.13, OR = 2.182 95% CI OR = 1.168–4.074 |
| Other, n (%) | 153 (82) | 172 (89.4) | |
| Not known, n | 14 | 11 | |
| Healthcare profession | N = 33 | N = 17 | |
| Student, n (%) | 4 (12) | 0 | |
| Nurse, n (%) | 21 (64) | 10 (58.7) | |
| Medical doctor, n (%) | 0 | 1 (5.9) | |
| Psychologist, n (%) | 3 (9) | 3 (17.6) | |
| Pharmacist or assistant, n (%) | 2 (6) | 0 | |
| Social aid or educator, n (%) | 3 (9) | 2 (11.8) | |
| Physiotherapist, n (%) | 0 | 1 (5.9) |
Profession
Among FND patients (200), 17 were students, 149 were professionally active, 11 were already retired, and 23 were receiving a disability allowance. The distribution among controls (200) was similar: 175 were professionally active at the time of admission; nine were retired, 12 were receiving a disability allowance, and four were students. For 14 FND patients (three active, six retired and five with disability allowance) and 11 controls (seven retired, four with disability allowance) no detailed information about profession was available.
The proportion of healthcare professionals in FND patients was 18%, which was significantly higher than among controls, where the proportion was 10.6% (p = 0.019, OR 2.182, 95% CI OR 1.168–4.074). Most healthcare professionals in both cohorts were nurses (21/33 among FND, 10/17 among controls). Four FND patients were students: two of them were nurses in education, one was a medical student, and one was a student of psychology.
Focusing on disability allowance, we did not find a significantly higher proportion of FND patients compared to controls (p = 0.076, OR 2.035, 95% CI OR 0.983–4.123).
More details in Table 1 and Figures 1 and 2.
FIGURE 1.
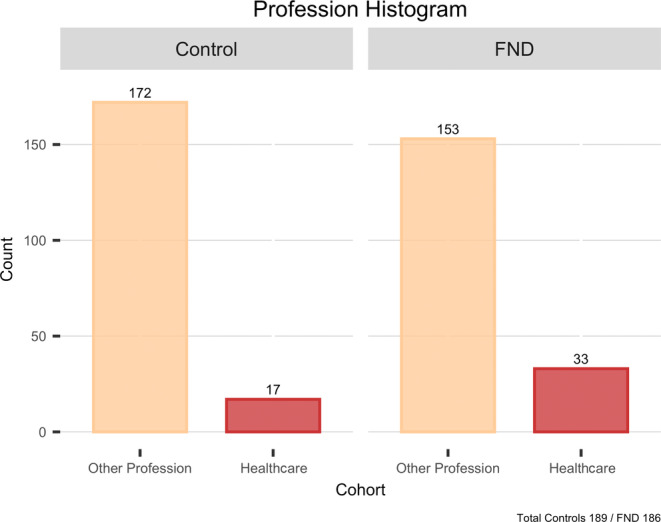
Category of profession among the two cohorts. FND, functional neurological disorder.
FIGURE 2.
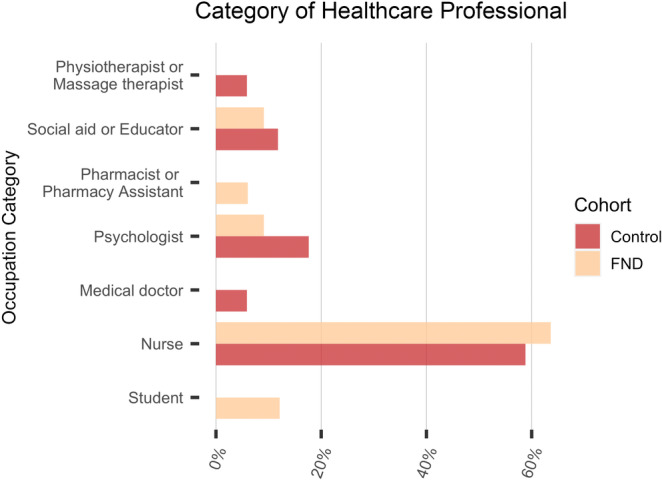
Healthcare profession categories among the two cohorts. FND, functional neurological disorder.
Clinical details of the FND cohort
The majority of the FND cohort had motor symptoms (140/200); 78 had only motor symptoms, while 62 had concomitant sensory deficits. Sensory symptoms as unique dysfunction affected 19 patients. A total of 41 patients had primarily functional seizures: 17 were diagnosed exclusively with this disorder, while 24 also had sensorimotor symptoms.
Focusing on other neurological symptoms (59/200), 16 patients reported chronic dizziness. In one case, a concomitant diagnosis of postural‐perceptual persistent dizziness was made. Visual disturbance (15 patients) and cognitive impairments (memory difficulties or mental fatigue, nine patients) were also indicated as additional manifestations. Sixty‐five patients among the whole cohort had a concomitant psychiatric diagnosis: depression was reported in 26 patients, anxiety or panic disorder in 18, and mixed anxiety‐depressive disorder in seven. Further, FND patients frequently reported chronic pain (65/200) and headache (59/200). More details are provided in Table 3 and Figure 3.
TABLE 3.
Clinical details of patients with functional neurological disorder.
| Symptoms | Count (Total 200) n (%) |
|---|---|
| Main symptoms | 200 |
| Only motor | 78 (39) |
| Only sensory | 19 (9.5) |
| Sensorimotor | 62 (31) |
| Functional seizures | 17 (8.5) |
| Mixed | 24 (12) |
| Motor symptoms | 157 |
| Tremor | 17 (10.8) |
| Dystonia | 12 (7.6) |
| Gait disorder | 15 (9.5) |
| Weakness | 65 (41.4) |
| Myoclonus | 18 (11.4) |
| Mixed | 30 (19.1) |
| Sensory symptoms | 95 |
| Hemisyndrom left | 35 (36.9) |
| Hemisyndrom right | 29 (30.5) |
| Other a | 31 (32.6) |
| Other neurological symptoms | 59 |
| Motor | 5 (8.5) |
| Visual | 15 (25.4) |
| Cognitive | 9 (15.2) |
| Dizziness | 16 (27.1) |
| Speech troubles | 8 (13.6) |
| Other b | 6 (10.2) |
| Chronic pain (pain lasting >3 months) | 65 |
| Diffuse | 35 (55.4) |
| Upper limb | 5 (7.7) |
| Under limb | 3 (4.6) |
| Back pain | 15 (23.1) |
| Unilateral | 3 (4.6) |
| Other c | 3 (4.6) |
| Headache | 59 |
| Migraine | 39 (66.1) |
| Cervical/myofascial | 3 (5.1) |
| Functional/tensional | 8 (13.6) |
| Unspecified | 9 (15.2) |
| Concomitant psychiatric diagnosis | 65 |
| Depression | 26 (40) |
| Anxiety/panic disorder | 18 (27.7) |
| Anxious‐depressive disorder | 7 (10.8) |
| Post‐traumatic stress disorder | 5 (7.7) |
| History of burnout | 2 (3.1) |
| Bipolar disorder | 1 (1.5) |
| Schizophrenia or psychotic episodes | 3 (4.6) |
| Attention deficit hyperactivity disorder | 3 (4.6) |
| No therapy | 24 (12) |
| Single therapy | 70 (35) |
| Physiotherapy only | 51 |
| Psychotherapy only | 13 |
| Antidepressant | 6 |
| Double therapy | 71 (35.5) |
| Physiotherapy + psychotherapy | 43 |
| Physiotherapy + antidepressant | 9 |
| Psychotherapy + antidepressant | 19 |
| Triple therapy | 35 (17.5) |
Hypoesthesia in different body parts (e.g., patchy, two limbs on both sides), n = 13; episodic sensory dysesthesia, n = 3 (both hands, n = 1; under limbs, n = 2); hypoesthesia of a single body part (e.g., foot, leg), n = 3; painful dysesthesia, n = 1; thermal dysesthesia, n = 1; functional blindness, n = 1; paresthesia foot sole, n = 1; hypopallestesia right side of the face, n = 1; unspecified, n = 7.
Small‐fiber polyneuropathy, n = 2; Parkinson's disease n = 1, unspecified polyneuropathy, n = 1; unspecified, n = 2.
Pelvic pain syndrome, n = 1; chronic jaw pain, n = 1; chronic diffuse abdominal pain, n = 1.
FIGURE 3.
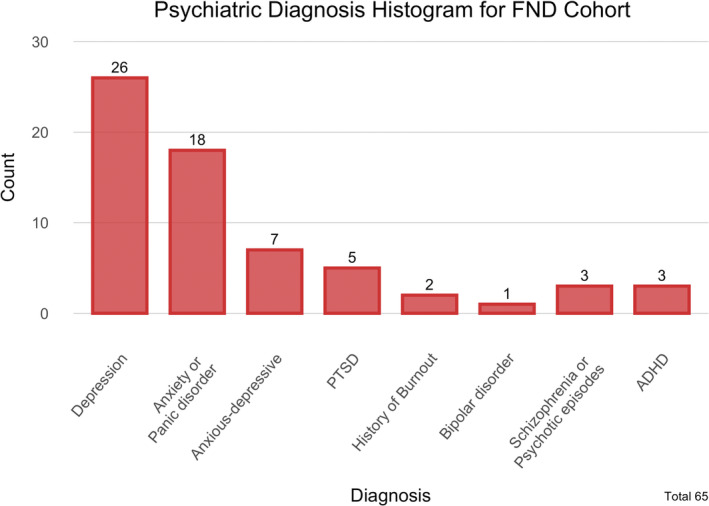
Concomitant psychiatric diagnosis among functional neurological disorder (FND) patients. ADHD, attention deficit hyperactivity disorder; PTSD, post‐traumatic stress disorder.
Clinical details of the neurological control cohort
Multiple sclerosis was the most frequent cause for a referral to the neurological department (n = 59), followed by vascular diseases (n = 35), epilepsy (n = 31) and headache or migraine (n = 34), which were similarly represented. Among vascular diseases, the most frequent diagnosis was stroke or transient ischemic attack. More detailed information is provided in Table 2 and Figure 4.
TABLE 2.
Diagnoses in neurological controls.
| Diagnosis | Count (Total 200) n (%) |
|---|---|
| Vascular disease | 36 (18) |
| Stroke | 22 |
| Transient ischemic attack | 4 |
| Sinusvenenthrombose | 2 |
| Carotid stenosis | 1 |
| Carotid dissection | 3 |
| Other | 4 |
| Epilepsy | 32 (6) |
| Headache | 35 (17.5) |
| Migraine | 24 |
| Cluster | 2 |
| Other | 3 |
| Unspecified | 6 |
| Multiple sclerosis | 59 (29.5) |
| Sleep disorder | 8 (4) |
| Intracerebral lesion | 7 (3.5) |
| Unclear lesion | 2 |
| Undifferentiated connective tissue disease | 1 |
| Metastasis | 1 |
| Meiningeoma | 1 |
| Multiple lesions | 2 |
| Sensory disorder a | 4 (2) |
| Other b | 19 (9.5) |
Paresthesia without clear etiology at first consultation.
Anti‐NMDA receptor encephalitis, n = 1; myasthenia gravis, n = 1; traumatic brain injury, n = 1; demyelinating polyneuropathy, n = 1; tickborne encephalitis, n = 2; Guillain‐Barré syndrome, n = 1; spastic paraplegia type 4, n = 1; idiopathic Parkinson disease, n = 2; idiopathic intracranial hypertension, n = 2; essential tremor, n = 1; recurrent immune reconstitution inflammatory syndrome with neurological symptoms, n = 1; mild cognitive impairment, n = 1; chronic pain syndrome, n = 1; spinal discherniation, n = 1; tinnitus, n = 1; benign paroxysmal positional vertigo, n = 1.
FIGURE 4.
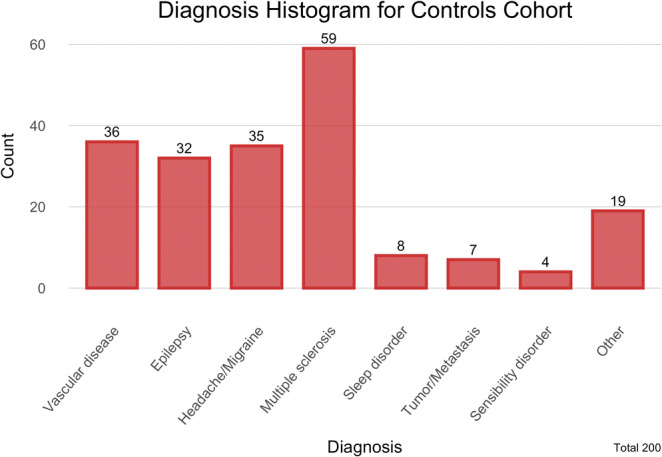
Diagnosis neurological controls.
DISCUSSION
This case–control study found significantly more healthcare professionals (18%) among 186 FND subjects than among a group of 189 age‐ and gender‐matched controls (10.6%) who had another neurological disease. This confirms a link between employment in a healthcare profession and FND and we suggest that working as a healthcare professional is a risk factor for the development of FND, possibly due to exposure to a “model” of neurological symptoms, either in the healthcare professional's daily work life or during their training/education.
In line with this disease‐modeling hypothesis, one study found that 66% of patients experiencing functional seizures had previously personally witnessed a seizure, whereas only 11% of patients experiencing epileptic seizure had done [15]. Likewise, a significantly higher proportion of functional movement disorder patients reported having witnessed symptoms similar to their own in family members or friends compared to controls with another movement disorder [16]. In other functional disorders (more broadly designated “medically unexplained symptoms”), exposure to previous illness through witnessing a parent (more often a father) being ill, was associated with later hospital admission for unexplained symptoms [17]. Even though our study did not directly measure the amount of exposure, we formulate the hypothesis that a certain degree of knowledge about neurological symptoms acquired in training and work as a healthcare professional may play a role. Indeed, we only classified as healthcare professionals those individuals who had contacts with patients and excluded workers in the healthcare system with no contact with patients, for example, health insurance employees.
An alternate explanation for the association between working in a healthcare profession and FND may be the unbalanced male/female ratio usually found in FND cohorts, as there are more females working in healthcare professions. Indeed, statistics from 2019 indicate that 74% of the jobs in the Swiss healthcare system were held by women [30] as reported by the Swiss Federal statistical office. The case series presented here, however, carefully matched subjects for gender and age and, with previous power calculation, was able to detect a significant difference between the two cohorts, strongly suggesting that gender does not account for this association.
Looking at type of healthcare profession, nurses were the most highly represented category among both cohorts. Different reasons underlie this finding. Firstly, the World Health Organization calculated that nurses comprise more than 50% of all global healthcare providers, making them the largest category among healthcare professionals in 2020 [31]. In the United States and Canada, there were four nurses for each doctor in 2006 [32]. Secondly, nurses usually have more contact with patients than any other category of healthcare professional, especially in tertiary care settings, possibly leading to a stronger effect of exposure [33], and in keeping with the proposed disease‐modeling hypothesis.
Another factor, however, must be considered besides disease modeling, namely, exposure to chronic and acute stress. Emotional, physical, or psychological stress are considered risk factors for FND [3, 5, 34] and, in fact, healthcare professional is one of the most stressful job categories. Among nurses, lower levels of recognition and shift working also explain in part the higher rate of moral distress compared to other professional groups within the healthcare system [33, 35]. In addition to work‐related stress, previous exposure to childhood trauma and adverse life events may play a role, with some studies reporting high rates of lifetime adversities among healthcare workers [36]. The association found between working in healthcare professions and FND may thus be mediated by a common stress exposure, disease modeling, or a combination of both. Only further prospective studies considering these factors will be able to disentangle this issue.
Finally, it could be hypothesized that certain personality traits, behavior styles and emotional intelligence drive the choice to choose healthcare as a profession. In fact, some common personality traits have been found among all healthcare professionals, such as low levels of neuroticism and high prevalence of agreeable, cooperative, and self‐directed traits [37], whereas differences have been noted among different healthcare professions regarding behavior style and emotional intelligence, including coping and external locus of control [37]. These factors may constitute vulnerability to develop FND but the literature on the topic is still scarce and more studies are warranted before an association can be demonstrated.
Demographics
Young, female individuals were more frequent in our cohort of cases, as reported also by other authors [5, 28]. The fact that females seem to be more susceptible to FND may relate to greater bodily awareness, higher propensity to express bodily distress, as well as a tendency to look for medical consultation on presence of symptoms [26]. Furthermore, higher abuse rates, and potential genetic, personality and hormonal predisposing factors may represent vulnerabilities in women [38, 39]. At the same time, the historical association of functional disorders and female gender may represent a bias during the diagnostic process, as clinicians may be more prone to make a diagnosis of FND if the patient is young and female while extending the investigation for differential diagnosis in an older male patient [38].
Disability
We did not find a higher rate of patients receiving disability allowance among FND cases compared to controls, in contrast to some authors who did describe such an association [7, 9]. A peculiarity of our sample was that several participants in the control cohort had a neurodegenerative disease, leading to follow‐up consultations and higher rates of receiving disability benefits. By contrast, in the FND cohort we enrolled only new referrals of FND patients, meaning that several of them were receiving disability allowance even before being diagnosed with a functional disorder. Often, FND patients present with unexplained symptoms for a long period while being misdiagnosed or not evaluated by specialists, leading to greater emotional distress and a chronic course of the disease [7, 10].
Clinical details
The most frequently reported category of symptom was motor symptoms. Functional seizures were often recorded as well, as a single manifestation or combined with motor or sensory disorders. Our findings were similar to those of other series, confirming a recurrent clinical presentation [2, 26, 28, 40]. Similarly, chronic pain was often an additional clinical aspect of FND patients' symptomatology. These features were comparable with those of other series [7, 40]. Although it is not possible to establish with certainty if the psychiatric condition occurred before or after the diagnosis of FND, its concomitant presence underlines the impact the disease can have on patients' general health and on the social and healthcare system. Our results identified a rate of concomitant psychiatric diagnosis in FND patients similar to that observed with common neurological disorders such as Parkinson's disease, multiple sclerosis, or stroke [41, 42, 43]. In those conditions, psychiatric comorbidity may lead to reduced treatment adherence, worsening of symptoms and decreased quality of life [44, 45, 46]. Therefore, we consider the observed prevalence in the FND cohort as remarkable and emphasize the need to target this aspect in treatment plans.
It is also remarkable that for 109 patients, who were then excluded from the analysis, an FND diagnosis could not be confirmed after first consultation, even when referred to our specialized clinic. At the same time, four controls were diagnosed at first consultation in the general outpatient department with unclear sensory disorder, with paresthesia reported as the main symptom, leaving functional disorder as a possible differential diagnosis. This underlines how relevant and helpful further research into the development of a specific diagnostic tool for FND would be, and that more research into biomarkers is needed.
Strengths and limitations
The major strength of our study is its sample size. According to power analysis based on previous studies [21, 26, 27, 40], we can be confident that the difference we detected with moderate effect size is of significance and as we carefully matched cases and controls for age and gender, we are confident that these two factors do not confound our main finding of an association between FND and healthcare employment.
A major limitation of our study is its retrospective nature. Indeed, this retrospective design did not allow us to record what kind of patients and symptoms the healthcare workers were exposed to and for how long. It was not possible to evaluate if patients were exposed to previous diseases with phenotypically similar features than their specific functional neurological disorder. Additionally, other possible risk factors (e.g., traumatic life experiences) were not considered. However, to narrow down the possibility that healthcare workers, as defined in our study, were either exposed to patients or has minimal medical knowledge, we excluded social workers and personnel working only in administrative medical institutions. Also, our data were collected through meticulous work, with particular attention paid to descriptive reports indicating social status and profession. We did not limit our research to an administration platform, but we thoroughly investigated patients' medical charts to obtain the most trustworthy information.
We also had to exclude 138 patients who did not provide consent to use their clinical data for research purposes. This might have introduced a bias, for example, skewing the data towards a greater number of healthcare professionals providing consent or the inverse. This potential bias should however affect both cases and controls. It could even be hypothesized that, as FND is still stigmatized [10], patients working in the healthcare sector might have withdrawn consent, not wanting to appear in FND research, thus reducing an effect of a higher rate of healthcare professionals in the FND group as compared to controls.
Finally, our definition of gender was another potential limitation. As we based our analysis on self‐reported gender at the time of admission, the presence of transgender individuals might lead to bias in our analysis, as biological sex would not be reflected.
CONCLUSION
In conclusion, this case–control study confirmed a higher prevalence of employment in healthcare professions in FND patients (18%) compared to patients with other neurological conditions (10.6%). As we limited the definition of healthcare employment to individuals who potentially had direct contact with patients, our findings support the hypothesis that disease modeling may be a mechanism influencing the development of FND. Other general mechanisms may explain these results, in particular, exposure to stress should be mentioned, as healthcare providers are subject to stress linked to the emotional burden and physical challenges (e.g., shift working) associated with the profession. To better understand why this higher rate is present, prospective studies should attempt to measure exposure to patients with neurological symptoms as well as general stress measures. This will help disentangle the influence of disease modeling versus a general effect of stress. New studies investigating rates of FND among healthcare professionals before and after the COVID‐19 pandemic may also help elucidate the role of stress. These findings should not be interpreted in the sense that disease modeling suggests in any way a conscious mimicking of symptoms to which the patients may have been exposed. This simplification may have negative consequences in perpetuating stigma around FND, implying that knowing how a disease presents renders it possible to feign. Instead, we suggest that more research is needed to refine our knowledge of the risk factors for FND, including being exposed to models, as recent findings in fundamental neuroscience research have shown how symptom production in FND may be linked to aberrant neuronal networks, including prior knowledge and predictive coding [22, 23]. A deeper knowledge in this area may improve treatment strategies in the future as well as prevention.
AUTHOR CONTRIBUTIONS
Giorgio Vanini: Conceptualization; writing—original draft; Writing—review and editing; investigation; formal analysis; visualization; methodology; project administration. Janine Bühler: Conceptualization; methodology; visualization; writing—review and editing; project administration; formal analysis; investigation. Samantha Weber: Conceptualization; methodology; writing—review and editing. Manuela Steinauer: Investigation; writing—review and editing; methodology; project administration. Selma Aybek: Conceptualization; methodology; writing—review and editing; project administration; funding acquisition; supervision.
FUNDING INFORMATION
This work was supported by the Swiss National Science Foundation (SNF Grant PP00P3_210997).
CONFLICT OF INTEREST STATEMENT
The authors report no competing interests.
ACKNOWLEDGEMENTS
Open access funding provided by Universite de Fribourg.
Vanini G, Bühler J, Weber S, Steinauer M, Aybek S. Healthcare employment as a risk factor for functional neurological disorder: A case–control study. Eur J Neurol. 2024;31:e16056. doi: 10.1111/ene.16056
DATA AVAILABILITY STATEMENT
Data available on request.
REFERENCES
- 1. Espay AJ, Aybek S, Carson A, et al. Current concepts in diagnosis and treatment of functional neurological disorders. JAMA Neurol. 2018;75(9):1132‐1141. doi: 10.1001/jamaneurol.2018.1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmad O, Ahmad KE. Functional neurological disorders in outpatient practice: an Australian cohort. J Clin Neurosci. 2016;28:93‐96. doi: 10.1016/j.jocn.2015.11.020 [DOI] [PubMed] [Google Scholar]
- 3. Carson AJ, Ringbauer B, Stone J, McKenzie L, Warlow C, Sharpe M. Do medically unexplained symptoms matter? A prospective cohort study of 300 new referrals to neurology outpatient clinics. J Neurol Neurosurg Psychiatry. 2000;68(2):207‐210. doi: 10.1136/jnnp.68.2.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miyasaki JM, Sa DS, Galvez‐Jimenez N, Lang AE. Psychogenic movement disorders. Can J Neurol Sci. 2003;30(SUPPL 1):S100. doi: 10.1017/s0317167100003292 [DOI] [PubMed] [Google Scholar]
- 5. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112(9):747‐751. doi: 10.1016/j.clineuro.2010.05.011 [DOI] [PubMed] [Google Scholar]
- 6. Ramsay N, Stone J, Fadiloglu K, et al. Functional neurological disorder: a common reason for a neurology inpatient referral. Eur J Neurol. 2023;30(12):3886‐3889. doi: 10.1111/ene.16003 [DOI] [PubMed] [Google Scholar]
- 7. Carson A, Stone J, Hibberd C, et al. Disability, distress and unemployment in neurology outpatients with symptoms “unexplained by organic disease”. J Neurol Neurosurg Psychiatry. 2011;82(7):810‐813. doi: 10.1136/jnnp.2010.220640 [DOI] [PubMed] [Google Scholar]
- 8. Carson A, Lehn A. Epidemiology. Handb Clin Neurol. 2016;139:47‐60. doi: 10.1016/B978-0-12-801772-2.00005-9 [DOI] [PubMed] [Google Scholar]
- 9. Rask MT, Rosendal M, Fenger‐Grøn M, Bro F, Ørnbøl E, Fink P. Sick leave and work disability in primary care patients with recent‐onset multiple medically unexplained symptoms and persistent somatoform disorders: a 10‐year follow‐up of the FIP study. Gen Hosp Psychiatry. 2015;37(1):53‐59. doi: 10.1016/j.genhosppsych.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 10. LaFaver K, Lang AE, Stone J, et al. Opinions and clinical practices related to diagnosing and managing functional (psychogenic) movement disorders: changes in the last decade. Eur J Neurol. 2020;27(6):975‐984. doi: 10.1111/ene.14200 [DOI] [PubMed] [Google Scholar]
- 11. Walzl D, Carson AJ, Stone J. The misdiagnosis of functional disorders as other neurological conditions. J Neurol. 2019;266(8):2018‐2026. doi: 10.1007/s00415-019-09356-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spagnolo PA, Johnson K, Hodgkinson C, Goldman D, Hallett M. Methylome changes associated with functional movement/conversion disorder: influence of biological sex and childhood abuse exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2023;125:125. doi: 10.1016/j.pnpbp.2023.110756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spagnolo PA, Norato G, Maurer CW, et al. Effects of TPH2 gene variation and childhood trauma on the clinical and circuit‐level phenotype of functional movement disorders. J Neurol Neurosurg Psychiatry. 2020;91(8):814‐821. doi: 10.1136/jnnp-2019-322636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Apazoglou K, Adouan W, Aubry JM, Dayer A, Aybek S. Increased methylation of the oxytocin receptor gene in motor functional neurological disorder: a preliminary study. J Neurol Neurosurg Psychiatry. 2018;89(5):552‐554. doi: 10.1136/jnnp-2017-316469 [DOI] [PubMed] [Google Scholar]
- 15. Bautista RED, Gonzales‐Salazar W, Ochoa JG. Expanding the theory of symptom modeling in patents with psychogenic nonepileptic seizures. Epilepsy Behav. 2008;13(2):407‐409. doi: 10.1016/j.yebeh.2008.04.016 [DOI] [PubMed] [Google Scholar]
- 16. Pellicciari R, Superbo M, Gigante AF, Livrea P, Defazio G. Disease modeling in functional movement disorders. Parkinsonism Relat Disord. 2014;20(11):1287‐1289. doi: 10.1016/j.parkreldis.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 17. Hotopf M, Wilson‐Jones C, Mayou R, Wadsworth M, Wessely S. Childhood predictors of adult medically unexplained hospitalisations: results from a national birth cohort study. Br J Psychiatry. 2000;176:273‐280. doi: 10.1192/bjp.176.3.273 [DOI] [PubMed] [Google Scholar]
- 18. Stamelou M, Cossu G, Edwards MJ, et al. Familial psychogenic movement disorders. Mov Disord. 2013;28(9):1295‐1298. doi: 10.1002/mds.25463 [DOI] [PubMed] [Google Scholar]
- 19. Nowak DA, Fink GR. Psychogenic movement disorders: aetiology, phenomenology, neuroanatomical correlates and therapeutic approaches. Neuroimage. 2009;47(3):1015‐1025. doi: 10.1016/j.neuroimage.2009.04.082 [DOI] [PubMed] [Google Scholar]
- 20. Shill H, Gerber P. Evaluation of clinical diagnostic criteria for psychogenic movement disorders. Mov Disord. 2006;21(8):1163‐1168. doi: 10.1002/mds.20921 [DOI] [PubMed] [Google Scholar]
- 21. McCormack R, Moriarty J, Mellers JD, et al. Specialist inpatient treatment for severe motor conversion disorder: a retrospective comparative study. J Neurol Neurosurg Psychiatry. 2014;85:895‐900. doi: 10.1136/jnnp-2013-305716 [DOI] [PubMed] [Google Scholar]
- 22. Edwards MJ, Adams RA, Brown H, Pareés I, Friston KJ. A Bayesian account of “hysteria”. Brain. 2012;135(11):3495‐3512. doi: 10.1093/brain/aws129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hallett M, Aybek S, Dworetzky BA, McWhirter L, Staab JP, Stone J. Functional neurological disorder: new subtypes and shared mechanisms. Lancet Neurol. 2022;21(6):537‐550. doi: 10.1016/S1474-4422(21)00422-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hull M, Parnes M. Tics and TikTok: functional tics spread through social media. Mov Disord Clin Pract. 2021;8(8):1248‐1252. doi: 10.1002/mdc3.13267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Müller‐Vahl KR, Pisarenko A, Jakubovski E, Fremer C. Stop that! It's not Tourette's but a new type of mass sociogenic illness. Brain. 2022;145(2):476‐480. doi: 10.1093/brain/awab316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Connell N, Nicholson TR, Wessely S, David AS. Characteristics of patients with motor functional neurological disorder in a large UK mental health service: a case‐control study. Psychol Med. 2020;50(3):446‐455. doi: 10.1017/S0033291719000266 [DOI] [PubMed] [Google Scholar]
- 27. Perry CG, Holmes KG, Gruber‐Baldini AL, et al. Are patients with psychogenic movement disorders more likely to be healthcare workers? Mov Disord Clin Pract. 2017;4(1):62‐67. doi: 10.1002/mdc3.12351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kenney C, Diamond A, Mejia N, Davidson A, Hunter C, Jankovic J. Distinguishing psychogenic and essential tremor. J Neurol Sci. 2007;263(1–2):94‐99. doi: 10.1016/j.jns.2007.06.008 [DOI] [PubMed] [Google Scholar]
- 29. R Core Team . R: a language and environment for statistical computing. 2021. Accessed January 8, 2023. https://www.R‐project.org/
- 30. Bundesamt für Statistik . Health—Pocket Statistics 2022 . 2022.
- 31. WHO . Health Workforce. State of the World's Nursing . 2020.
- 32. Guilbert JJ. The World Health Report 2006 . 2006.
- 33. Whitehead PB, Herbertson RK, Hamric AB, Epstein EG, Fisher JM. Moral distress among healthcare professionals: report of an institution‐wide survey. J Nurs Scholarsh. 2015;47(2):117‐125. doi: 10.1111/jnu.12115 [DOI] [PubMed] [Google Scholar]
- 34. Asadi‐Pooya AA, Emami M. Demographic and clinical manifestations of psychogenic non‐epileptic seizures: the impact of co‐existing epilepsy in patients or their family members. Epilepsy Behav. 2013;27(1):1‐3. doi: 10.1016/j.yebeh.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 35. Piko BF. Burnout, role conflict, job satisfaction and psychosocial health among Hungarian health care staff: a questionnaire survey. Int J Nurs Stud. 2006;43(3):311‐318. doi: 10.1016/j.ijnurstu.2005.05.003 [DOI] [PubMed] [Google Scholar]
- 36. Maunder RG, Peladeau N, Savage D, Lancee WJ. The prevalence of childhood adversity among healthcare workers and its relationship to adult life events, distress and impairment. Child Abuse Negl. 2010;34(2):114‐123. doi: 10.1016/j.chiabu.2009.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Louwen C, Reidlinger D, Milne N. Profiling health professionals' personality traits, behaviour styles and emotional intelligence: a systematic review. BMC Med Educ. 2023;23(1):120. doi: 10.1186/s12909-023-04003-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lidstone SC, Costa‐Parke M, Robinson EJ, Ercoli T, Stone J. Functional movement disorder gender, age and phenotype study: a systematic review and individual patient meta‐analysis of 4905 cases. J Neurol Neurosurg Psychiatry. 2022;93(6):609‐616. doi: 10.1136/jnnp-2021-328462 [DOI] [PubMed] [Google Scholar]
- 39. Kletenik I, Sillau SH, Isfahani SA, LaFaver K, Hallett M, Berman BD. Gender as a risk factor for functional movement disorders: the role of sexual abuse. Mov Disord Clin Pract. 2020;7(2):177‐181. doi: 10.1002/mdc3.12863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Delgado C, Kurtis M, Martin B, et al. Clinical and demographic characteristics of patients with functional movement disorders: a consecutive cohort study from a specialized clinic. Acta Neurol Belg. 2022;122(1):97‐103. doi: 10.1007/s13760-021-01648-8 [DOI] [PubMed] [Google Scholar]
- 41. Sparaco M, Lavorgna L, Bonavita S. Psychiatric disorders in multiple sclerosis. J Neurol. 2021;268(1):45‐60. doi: 10.1007/s00415-019-09426-6 [DOI] [PubMed] [Google Scholar]
- 42. Reijnders JSAM, Ehrt U, Weber WEJ, Aarsland D, Leentjens AFG. A systematic review of prevalence studies of depression in Parkinson's disease. Mov Disord. 2008;23(2):183‐189. doi: 10.1002/mds.21803 [DOI] [PubMed] [Google Scholar]
- 43. Medeiros GC, Roy D, Kontos N, Beach SR. Post‐stroke depression: a 2020 updated review. Gen Hosp Psychiatry. 2020;66:70‐80. doi: 10.1016/j.genhosppsych.2020.06.011 [DOI] [PubMed] [Google Scholar]
- 44. Tarrants M, Oleen‐Burkey M, Castelli‐Haley J, Lage MJ. The impact of comorbid depression on adherence to therapy for multiple sclerosis. Mult Scler Int. 2011;2011:1‐10. doi: 10.1155/2011/271321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bruce JM, Hancock LM, Arnett P, Lynch S. Treatment adherence in multiple sclerosis: association with emotional status, personality, and cognition. J Behav Med. 2010;33(3):219‐227. doi: 10.1007/s10865-010-9247-y [DOI] [PubMed] [Google Scholar]
- 46. Schrag A. Quality of life and depression in Parkinson's disease. J Neurol Sci. 2006;248(1–2):151‐157. doi: 10.1016/j.jns.2006.05.030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request.


