FIGURE 6.
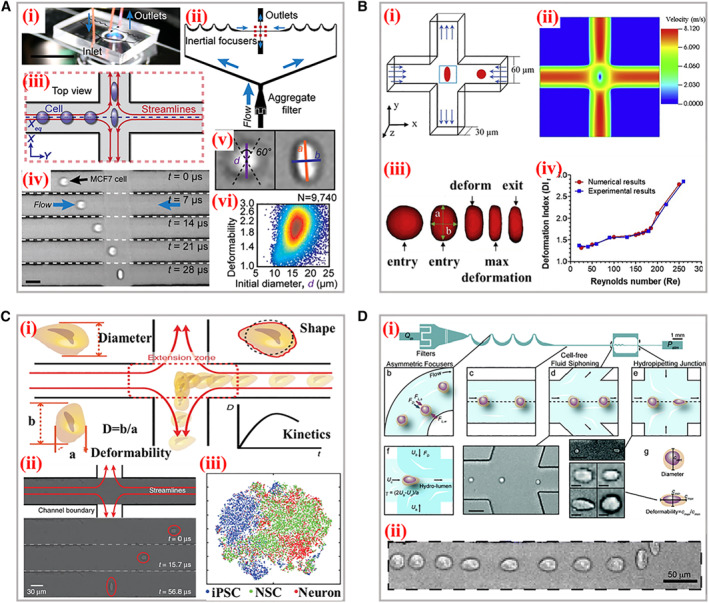
Typical working principle and applications of xDC. (A) (i) The photograph of the microscope‐mounted and fluid‐coupled microfluidic deformability cytometry device. (ii) The structures of microchannels focusing cells before delivering them to the stretching extensional flow. (iii) The scheme of the cellular deformation within an extensional flow previously aligned at an inertial focusing position. (iv) Microscopic images of a single cell entering the extensional region. (v) Definitions of the shape parameters extracted from images. (vi) Density scatter plot of deformability measurements of single human embryonic stem cells. Reproduced under terms of the CC‐BY license. 57 Copyright 2012, The Authors, published by National Academy of Sciences. (B) (i) Scheme of the cross‐flow channel geometry and cellular deformation under the velocity profiles. (ii) The velocity distribution within the extensional region without the cell present. (iii) The numerical simulation of the cellular shape changes through the cross‐flow channel. (iv) Comparison of the experimental and numerical deformation indexes of cells. Reproduced with permission. 58 Copyright 2020, Biophysical Society. (C) The schematic diagram of the microfluidic device for analysis of four parameters during cellular deformation process. (ii) High‐speed photography of the deformation. (iii) Visualization of physical phenotypic spaces occupied by iPSCs, NSCs, and neurons. Reproduced under terms of the CC‐BY license. 59 Copyright 2017, The Authors, published by Springer Nature. (D) (i) The scheme of the hydropipetting method possessing specific microchannel configurations for three working patterns. (ii) Overlaid images of a single cellular deformation including relaxing, and then deforming again in the extensional flow. Reproduced with permission. 60 Copyright 2013, The Royal Society of Chemistry.
