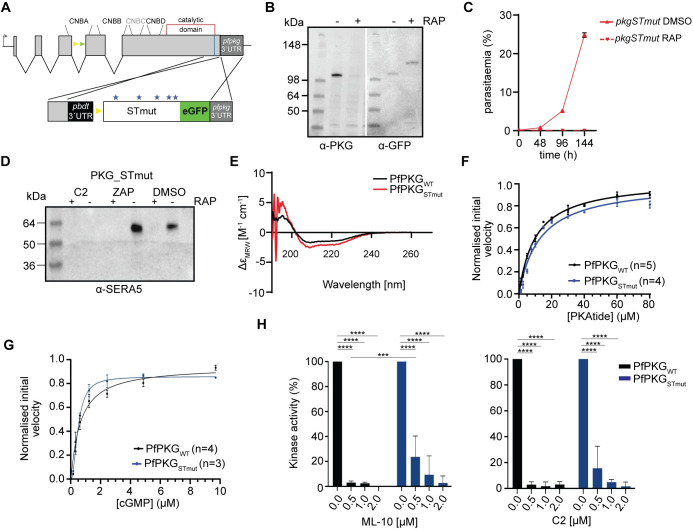
Fig 2. Kinase domain phosphomutants render PfPKG inactive in vivo without affecting kinase activity in vitro.
(A) Schematic of the approach used to create line pkgSTmut. Blue line indicates the position targeted by the gRNA. Stars depict the relative positions of the mutated amino acids. (B) Western blot analysis showing the distinct PKG versions in the absence or presence of rapamycin (RAP). (C) Replication rates of line pkgSTmut over three cycles. Error bars, ± S.D (n = 3). (D) Western blot of pkgSTmut parasites, showing no release of SERA5 P50 into culture supernatants of RAP-treated schizonts even upon addition of zaprinast (ZAP) consistent with impaired egress. (E) Circular dichroism (CD) spectra of recombinant PfPKGWT and PfPKGSTmut, showing no significant changes in secondary structure. (F and G) PKAtide Km determination and cGMP KA determination for recombinant PfPKGSTmut (in blue), relative to the control (PfPKGWT in black). Means ± SEM (see S1 Data). (H) Kinase activity of PfPKGWT and PfPKGSTmut in the presence of the established PKG inhibitors ML-10 (left panel) and C2 (right panel). Means ± SD, Two-way ANOVA with P<0.0001 (****) and P = 0.0009 (***).