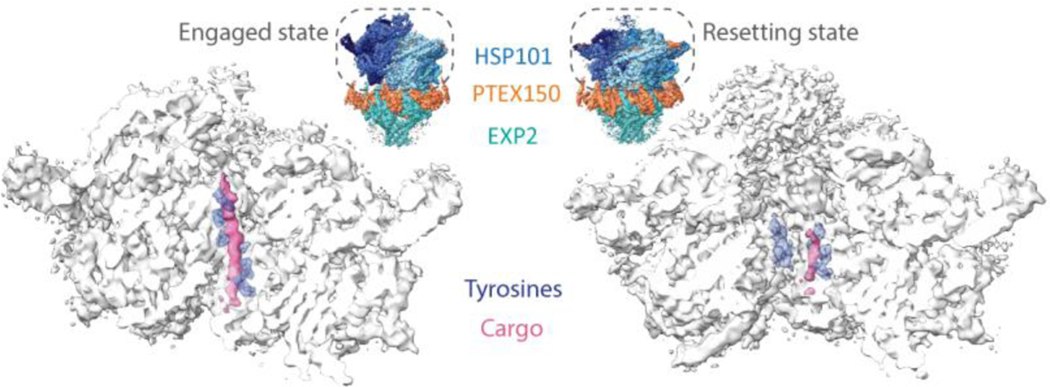
Figure 2 |. CryoEM of endogenous PTEX complex reveals native cargo.
CryoEM density maps of HSP101 from PTEX translocon in transparent white with cargo (pink) and interdigitating pore loop tyrosines (purple, stick representation) in the engaged and resetting states (EMD-8951, EMD-8952). Maps are bisected to show the endogenous cargo and pore loop tyrosines in the protein-unfolding channel of the HSP101 unfoldase. Comparing the positioning of these pore loops relative to the cargo between the two states suggests a model for the mechanisms by which HSP101 unfolds the cargo protein and threads it through the transmembrane channel of the translocon. Full PTEX complex structures are shown in the insets for context (PDB IDs: 6E10, 6E11).