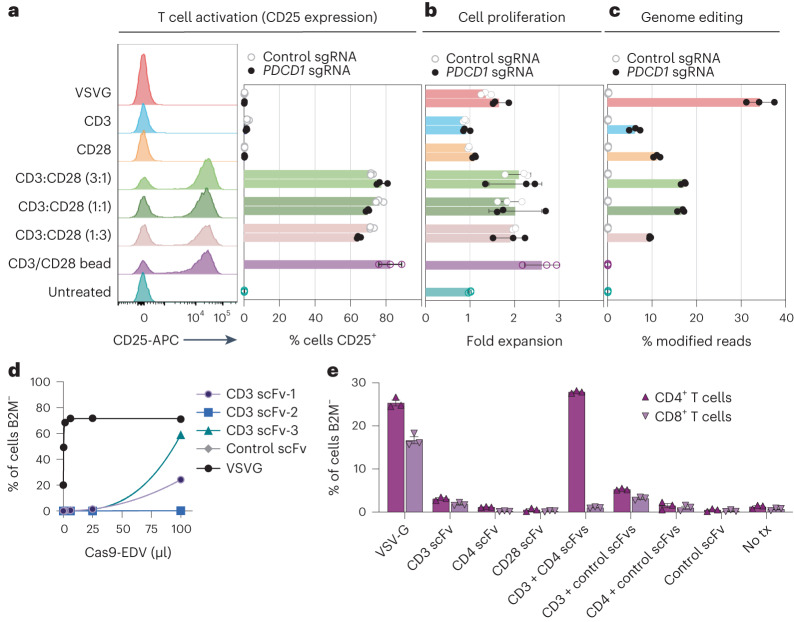
Fig. 3. Multiplexed antibody targeting and editing of primary human T cells.
a,b, Treating resting human T cells with Cas9-EDVs co-displaying CD3 and CD28 scFvs results in cellular activation (a) and proliferation (b) as measured by flow cytometry detection of CD25 3 days post treatment and fold expansion relative to the untreated T cell count, respectively. CD25 expression and cellular proliferation was observed for CD3/CD28 scFv Cas9-EDVs, regardless of whether they packaged Cas9 RNPs targeting PDCD1 or a non-targeting control. c, Genome editing 3 days post treatment, as detected by amplicon next-generation sequencing. For a–c, Cas9-EDVs were concentrated 62× and 50 μl was used to treat 30,000 resting T cells. CD3 scFv-1 and CD28 scFv-2 were tested. d, Screening the mono-display of additional CD3 scFv targeting molecules for B2M-targeted Cas9-EDVs on the Jurkat T cell line. B2M expression was assessed by flow cytometry 3 days post treatment. Cas9-EDVs were concentrated 15× and 50 μl was used to treat 30,000 Jurkat cells. e, Testing a panel of T cell-targeted, B2M-targeting Cas9-EDVs, displaying single or multiplexed scFv targeting molecules. Activated primary human T cells were treated with 1.38 × 108 Cas9-EDVs, displaying one or a combination of CD3 scFv-3, CD4 scFv-2, CD28 scFv-2 and a control scFv, and were assessed for B2M expression in CD4+ and CD8+ T cells by flow cytometry 6 days post treatment. Error bars, s.e.m.; n = 3 technical replicates were used in all experiments.