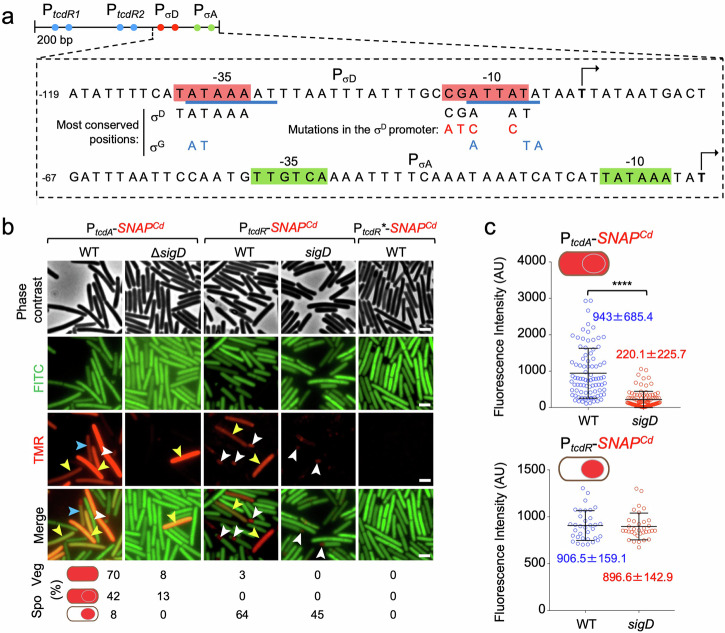
Fig. 3. σD regulates toxin production in whole sporangia but not in the forespore.
a Shows the regulatory region of the tcdR gene, with the −35 and −10 elements of the tandem tcdR-dependent promoters (PtcdR1 and PtcdR2, blue dots) and the σD- and σA-dependent promoters (red and green dots). The bases underlined in blue indicate a putative σG-dependent promoter. The most conserved positions for σD and σG-dependent promoters are shown as well as the point mutations introduced in the −10 region of the σD promoter (letters in red). Transcriptional start sites are indicated by broken arrows. Numbering is relative to the start site downstream of the σA-type promoter. b Microscopy analysis of C. difficile cells carrying fusions of the tcdA, tcdR and tcdR* (with point mutations in the σD-dependent promoter) promoters to SNAPCd in strain 630Δerm (WT) and in the ΔsigD mutant. The cells were collected after 24 h of growth in TY liquid medium, labeled with TMR-Star and examined by phase contrast and fluorescence microscopy to monitor SNAPCd production. The merged images show the overlap between the TMR-Star (red) and the auto-fluorescence (green) channels. The images are representative of the expression patterns observed for the different fusions in three independent experiments (see Methods). Yellow arrowheads point to vegetative cells with SNAPCd expression, white arrowheads point to sporulating cells with forespore-specific expression and blue arrowheads point to sporulating cells with whole sporangium expression. To score the indicated patterns in vegetative (Veg) or sporulating cells (Spo) the number of cells analyzed for each strain, n, was as follows: WT with PtcdA-SNAPCd, n = 856; sigD with PtcdA-SNAPCd, n = 1550; WT with PtcdR-SNAPCd, n = 670; sigD with PtcdR-SNAPCd, n = 3902; WT with PtcdR*-SNAPCd, n = 268. c Fluorescence intensity (in Arbitrary Units, AU) of the SNAPCd signal per sporangia for the tcdA fusion and in the forespore for the tcdR fusion, in the WT or in the ΔsigD mutant. The numbers in the panels represent the mean value ± the standard deviation. ****, p < 0.0001; no stars, non-significant differences (see Methods). Scale bar, 1 μm.