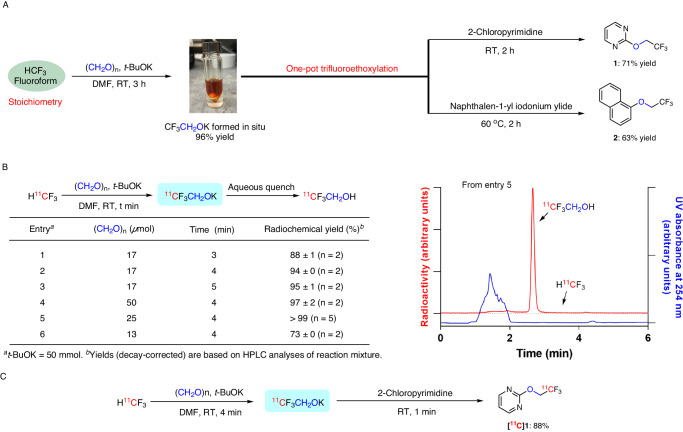
Fig. 2. Reactions of fluoroform and [11C]fluoroform.
A One-pot two-stage trifluoroethoxylations with fluoroform to give 2,2,2-trifluoroethyl ethers. Yields were determined with 19F NMR spectroscopy. (For more detail, see Supplementary Table 1 and Supplementary Fig. 1). B Optimization of conversion of [11C]fluoroform with paraformaldehyde into [2-11C]2,2,2-trifluoroethanol (left panel) and HPLC chromatogram of the crude reaction mixture from entry 5 (right panel). C Example of one-pot, two-stage synthesis of 2-[11C](2,2,2-trifluoroethoxy)pyrimidine.