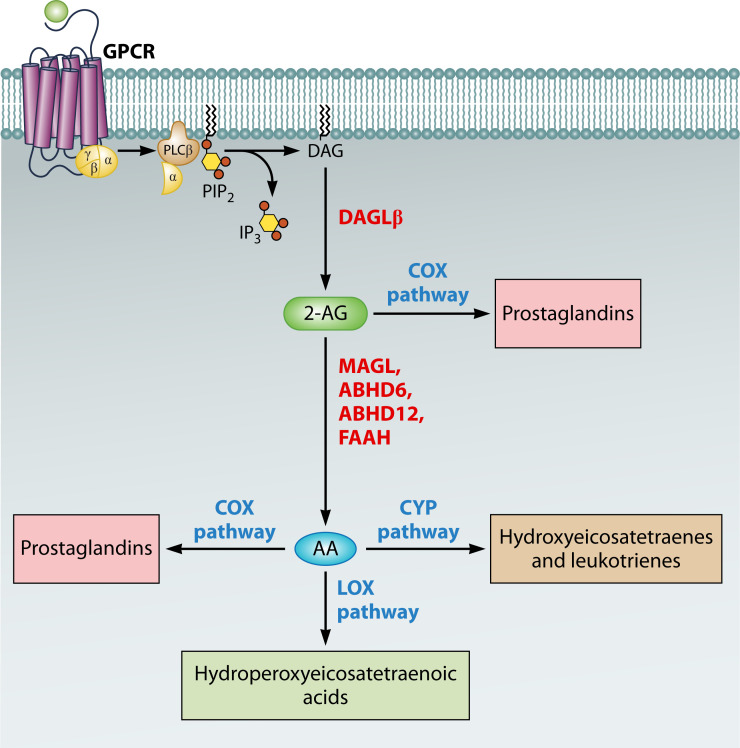
Fig 1.
Representation of the biosynthesis and degradation pathways of 2-AG. This diagram illustrates the metabolic pathways involved in the synthesis and breakdown of 2-arachidonoylglycerol (2-AG). Stimulation of G protein-coupled receptors (GPCR) activates Phospholipase C (PLC), leading to the release of diacylglycerol (DAG) and inositol triphosphate. DAG is subsequently converted into 2-AG. The degradation of 2-AG is mediated by several enzymes: monoacylglycerol lipase (MAGL), alpha/beta-hydrolase domain 6 (ABHD6), alpha/beta-hydrolase domain 12 (ABHD12), and fatty acid amide hydrolase (FAAH), resulting in the production of arachidonic acid (AA). AA serves as a precursor for the synthesis of various eicosanoids through the cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 oxidase (CYP) pathways. These pathways generate bioactive lipids, such as prostaglandins, leukotrienes, and hydroperoxyeicosatetraenoic acids, which are critical for the inflammatory response and cellular signaling. Additionally, COX enzymes can directly transform 2-AG into prostaglandins.