Abstract
Background
Acne inversa (AI) is a refractory inflammatory skin disease, and TNF-α plays an important role in the pathogenesis of AI. By blocking TNF-α, infliximab (IFX) has been proven to be a promising method.
Objectives
To explore the underlying mechanisms of IFX treatment in AI patients.
Methods
In this research, we integrated transcriptome sequencing data from the samples of our patients with AI and the GEO database. Ex vivo skin culture of AI patients was conducted to evaluate the efficacy of IFX treatment. Animal studies and cell experiments were used to explore the therapeutic effect and mechanism of IFX treatment.
Results
Both TNF-α and NLRP3 inflammasome-related pathways were enriched in skin lesions of AI patients and murine AI models. After IFX treatment, the NLRP3 inflammasome-related pathway was effectively blocked, and the IL-1β level was normalized in ex vivo AI skin explants and murine AI models. Mechanistically, IFX suppressed the NF-κB signaling pathway to lower the expression of NLRP3 and IL-1β in keratinocytes.
Conclusions
IFX treatment alleviated skin lesions in murine AI models and downregulated NLRP3 and IL-1β expression levels by inhibiting the NF-κB signaling pathway, which was helpful for understanding the mechanism of IFX therapy.
Keywords: Acne inversa, Infliximab, TNF-α, IL-1β, NLRP3
1. Introduction
Acne inversa (AI; OMIM #142690), also referred to as hidradenitis suppurativa, is a chronic inflammatory skin disease characterized by distinctive manifestations including painful nodules, abscesses, and sinus tracts, predominantly in areas with apocrine glands [1,2]. The underlying pathogenesis of AI is multifactorial and not completely understood. Recent findings suggest that various proinflammatory mediators and chemokines are involved in the inflammatory stage of AI [2]. Among these factors, tumor necrosis factor α (TNF-α) is an important and pleiotropic cytokine highly expressed in both the skin lesions and blood of AI patients [3]; it acts on several cell types, including keratinocytes, macrophages and T lymphocytes, and induces a wide range of chemokines [4,5]. The significance of TNF-α is further supported by patients responding to anti-TNF-α therapy [6].
Among TNF-α antibodies, adalimumab (ADA) is the only biologic drug approved by the US Food and Drug Administration (FDA) for patients with AI [7,8]. However, not all patients respond well to ADA [9]. Infliximab (IFX), another TNF-α inhibitor, is a chimeric (mouse/human) monoclonal antibody; it binds to soluble and transmembrane receptor-bound TNF-α and inhibits its activity [6]. Compared with ADA, IFX displayed a similar or even better efficacy in AI treatment, including reducing disease severity, improving quality of life and normalizing laboratory parameters [10,11]. Drug survival (the probability of continuing to receive therapy) was also comparable between IFX and ADA [11]. Therefore, IFX is another effective option for AI treatment by targeting TNF-α. Considering that TNF-α could trigger other inflammatory cytokines implicated in the development of AI, there is lack of data on the anti-inflammatory effects of IFX in the treatment of AI when TNF-α is blocked; therefore, it is essential to conduct the mechanistic studies on this basis.
The aim of this study was to investigate the changes in TNF-α-dependent inflammatory molecules and pathways when IFX is applied to treat AI. The study was designed to detect (i) the enrichment of pathways in the skin lesions of AI patients and murine AI models and (ii) the effect and molecular signature of IFX treatment in ex vivo human AI skin explants and keratinocytes in vitro and murine AI models.
2. Materials and methods
2.1. Clinical samples collection
This study was approved by the Medical Ethics Committee of the Chinese Academy of Medical Sciences Institute of Dermatology (2017-KY-015) and was performed according to the principles of the Declaration of Helsinki. Written informed consent was provided by all the individuals participating in the experiments. Tissue and serum samples from a cohort of AI patients (n = 7) and healthy controls (n = 7) were utilized for quantitative real-time PCR analysis (qRT-PCR), ELISA, ex-vivo skin culture treatments, and histopathological analyses. Based on the consideration of sample minimization, the number of samples we selected met the basic analysis requirements, and the data from GEO database (GSE148027, GSE154773) also expand our sample range. Briefly, inclusion criteria were: Adult patients (>18 years) with (a) duration for at least 4 years, (b) at least a Hurley II severity, (c) didn't receive biologics treatment and were off any other systemic treatment or topical agents for at least 4 weeks. AI patients with a history of diabetes, hyperuricemia, gout, hyperlipidemia, hypertension and coronary heart disease were excluded. The detailed information of AI patients and healthy individuals were shown in Supplementary Table 1.
2.2. Antibodies and reagents
The following antibodies were used: TNF-α (Novus, NBP1–19532SS), IL-1β (Novus, NB600-633), NLRP3 (Novus, NBP2–12446SS), IκB (Cell Signaling Technology, #9936), phospho-IκB (Cell Signaling Technology, #9936), β-ACTIN (Santa Cruz, sc-47778), Goat anti-Rabbit IgG Alexa Fluro 594 (Life technology, A147701), Peroxidase-labeled anti-rabbit IgG (KPL, #074–1516). The complete culture medium used in the experiments was Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 % fetal bovine serum (FBS; Life Technologies). Recombinant human TNF-α was purchased from PeproTech (Rocky Hill, NJ, USA). Anti-TNF-α antibody IFX and NF-κB inbibitor BAY11-7082 were obtained from MedChem Express (MCE, NJ, USA).
2.3. Ex-vivo skin culture treatment
Six-millimeter skin punch biopsies were taken from actively inflamed AI lesions [[12], [13], [14]]. The biopsies were instantly transferred into a transwell system (Corning, USA) and placed into an insert of a 12-well plate with the epidermis pointing upwards in the air–liquid interface. The ex vivo explants were cultured for 24 h at 37 °C in the presence of 5 % CO2 in a humidified atmosphere. The culture media consisted of Iscove's Modified Dulbecco's medium (Gibco, Carlsbad, USA) supplemented with 0.5 % human AB serum (TCS Bioscience) in the presence of IFX or PBS, respectively, and was pipetted below the insert. We found that 20 μg/mL of IFX effectively alleviated the inflammatory response in the ex-vivo skin culture model through preliminary experiments. Consequently, this concentration of IFX was employed in subsequent experiments. After treatment, the biopsies were fixed in 4 % formalin or embedded with optimal cutting temperature compound (OCT) and the culture medium was collected, frozen and stored at −80 °C for further study. The culture supernatant was performed utilizing Luminex multifactor detection technology, facilitated by LabEx (Shanghai, China) and ELISA assay.
2.4. Cell culture and treatment
The human immortalized keratinocyte line HaCaT (ATCC, Manassas, Virginia, USA) was cultured with Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 % fetal bovine serum (FBS; Thermo Fisher Scientific, USA) in an atmosphere of 5 % CO2 at 37 °C. HaCaT line was authenticated using STR analysis and was negative for mycoplasma contamination. HaCaT cells were firstly incubated with BAY11-7082 (10 μM) or IFX (20 μg/mL) for 1 h. Then, TNF-α (20 ng/mL) was added in the media with the cells continuing to incubate in the presence of BAY11-7082 or IFX for an additional 24 h. Following treatment, both cells and their supernatants were collected for subsequent analysis by using qRT-PCR, Western blot and ELISA.
2.5. Animal studies
All animal experimental procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Ethical Board of Nanjing University (IACUC-2204017). To generate the keratinocyte-specific Ncstn conditional-knockout (NcstnΔKC) mice, we first crossed B6; 129-Ncstntm1Sud/J mice (Jackson laboratory, Bar Harbor, ME, USA) with Tg (Krt14-cre/ERT)20Efu (Krt14 CreER) mice to produce Ncstn flx/+; Krt14 CreER ± mice. Then, Ncstn flx/+; Krt14 CreER ± males were crossed with Ncstn flx/flx females to obtain Ncstn flx/flx; Krt14 CreER ± mice. Age- and sex-matched 6–8-wk-old mice were randomly assigned to animal studies.
According to our prior research [15], under tamoxifen (20 mg/kg) (Sigma-Aldrich, St. Louis, MO, USA) administration once daily for 5 consecutive days, murine AI models were acquired. Three weeks later, murine AI models were performed for blocking antibody experiments. The dosage and injections strategy of IFX in current animal experiments were referenced to previous literatures on murine models [16,17]. The murine AI models received IFX (5 mg/kg) (MCE, NJ, USA) or isotype control (clone HRPN, BioXCell) once a week for two weeks by intraperitoneal injection. On day 48th post to the initial injection of tamoxifen, mice were sacrificed and skin lesions and serum were excised for histopathological analysis and ELISA assay (TNF-α and IL-1β).
2.6. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
qRT-PCR was performed in a LightCycler480 II Detection System (Roche, Switzerland) with ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co.,ltd) to determine gene expression according to the manufacturer's protocol. β-actin was used as the internal control for gene expression. The qRT-PCR primers were synthesized by Life Technologies, and the primer sequences were shown in Supplementary Table 2.
2.7. Western blot
Protein extracts from HaCaT were prepared using RIPA lysis buffer containing a protease inhibitor mixture (Sigma-Aldrich) at a 1:100 dilution, and the protein concentrations were assayed with a BCA protein assay kit (Beyotime, Nantong, China). The proteins were separated with 8–20 % glycine-SDS-PAGE and transferred to PVDF membranes for further experiments with the indicated antibodies. Normalization was performed by blotting the same membrane with an antibody against β-ACTIN.
2.8. Immunostainings
Sections from paraffin-embedded skin tissues were stained with hematoxylin-eosin (H&E) for histopathological study. Cryo- or paraffin sections were stained according to standard procedures and images were taken with an Olympus confocal microscope (FV1000, Olympus, Tokyo, Japan) and an Olympus microscope (BX53, Olympus, Tokyo, Japan). The skin sections were incubated with corresponding primary and secondary antibodies. For immunofluorescence, cryo-sections were counterstained with nuclear staining DAPI (Sigma-Aldrich). Skin sections stained with matched isotype controls were used as negative controls.
2.9. Statistical analysis
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway was conducted to determine the significant pathways through DAVID Bioinformatics Resources (https://david.ncifcrf.gov/). Moreover, gene set enrichment analysis (GSEA) was used to identify the key pathways and core genes during the development of AI. The results are expressed as standard error of mean (SEM). Data was statistically analyzed using Prism software (GraphPad Software Inc. La Jolla, CA, USA) and assessed for normality or homogeneity of variance. Differences between two groups were evaluated using the two-tailed Student's t-test. A value of P < 0.05 was considered significant; ns = not significant. Differentially expressed genes (DEGs) were identified by the following criteria: fold change ≥2 or ≤ 0.5 and P value <0.05.
3. Results
3.1. Aberrant upregulation of TNF-α in the skin lesions of AI patients and murine AI models
Transcriptome sequencing analysis based on the GEO database (data from GSE148027) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis indicated that the TNF signaling pathway was significantly enriched in AI patients (Fig. 1a). In our study, five AI patients were enrolled, and a clinical diagnosis was established according to AI criteria [18]. Immunohistochemistry (IHC) staining and ELISA indicated the upregulation of TNF-α levels in the skin lesions and serum of AI patients, respectively (Fig. 1b and c). Given the embryonic lethality of Ncstn-null mice, we developed a specialized mouse strain featuring keratinocyte-specific Ncstn knockout to mimic the clinical characteristics of AI, which was subsequently referred to as the murine AI model (Fig. 1d and Fig. S1). Transcriptome sequencing and KEGG analysis revealed the pivotal role of the TNF signaling pathway in murine AI models versus normal mice (Fig. 1e). Furthermore, TNF-α levels were elevated in skin lesions and serum of murine AI models when compared to normal mice (Fig. 1f–h).
Fig. 1.
The TNF signaling pathway was significantly enriched in the skin lesions of AI patients and murine AI models. (a) The top 22 inflammation-related KEGG pathways enriched from transcriptome sequencing in the skin of AI patients versus healthy individuals (GSE148027). n = 18 AI patients and n = 8 healthy individuals. (b) Skin sections from AI patients and healthy individuals were examined following H&E and TNF-α staining by IHC. Scale bar = 100 μm for H&E staining, Scale bar = 25 μm for IHC. (c) TNF-α levels in the serum of AI patients and healthy individuals were detected using ELISA analysis. AI patients (n = 7) and healthy individuals (n = 7) for panels (b–c). (d) Schematic diagram of murine AI model construction and representative images of normal mice and murine AI models harvested on day 48 after the initial tamoxifen treatment (20 mg/kg). (e) The top 18 enriched KEGG pathways identified from genes significantly increased in the skin lesions of murine AI models compared to normal mice. n = 4 mice per group. (f) Skin sections from normal mice and murine AI models were examined following TNF-α expression level by IHC analysis. Scale bar = 100 μm. (g–h) TNF-α expression level in the skin or serum of normal mice and murine AI models by qRT‒PCR (g) and ELISA (h). n = 8 mice per group for panels (d, f-h). *P < 0.05, **P < 0.01.
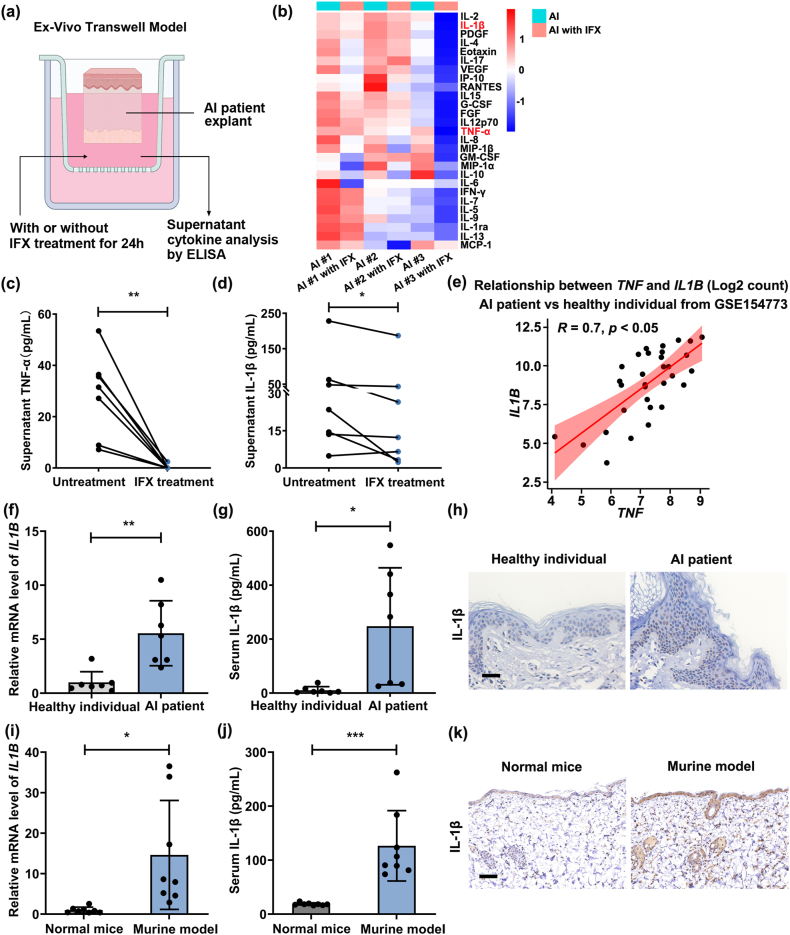
3.2. Infliximab induced downregulation of IL-1β expression
AI explants were cultured with IFX or PBS for 24 h, after which the culture supernatant was collected for the quantification of cytokines (Fig. 2a). The heatmap exhibited a comprehensive profiling of 27 cytokines in the culture supernatant of AI explants with IFX or PBS treatment by Luminex multifactor detection technology (Fig. 2b). Notably, the results from ELISA for TNF-α and IL-1β corroborated the trends after IFX treatment observed through Luminex analysis (Fig. 2c and d). Furthermore, through an analysis of data from GSE154773, we substantiated a positive correlation between the expression of TNF and IL1B (R = 0.7, P < 0.05) (Fig. 2e). Elevated mRNA and protein levels of IL-1β were detected within the lesional skin and serum of both AI patients (Fig. 2f–h) and the murine AI models (Fig. 2i–k).
Fig. 2.
Infliximab treatment decreased TNF-α and IL-1β expression levels. (a) Schematic diagram of the ex vivo Transwell model. (b) Cluster analysis of cytokines in the culture supernatant of AI patient lesions via Luminex multifactor detection technology. n = 3 patients. (c) TNF-α expression levels were detected in the culture supernatant of AI patient lesions treated with IFX (20 μg/mL) or PBS by ELISA. (d) ELISA was applied to detect IL-1β expression levels in the culture supernatant of AI patient lesions treated with IFX (20 μg/mL) or PBS. n = 7 AI patients for panels (c–d). (e) Correlation analysis of TNF and IL1B levels in the skin of AI patients and healthy individuals (data from GSE154773). n = 22 AI patients and n = 10 healthy individuals. (f–h) The mRNA and protein levels of IL-1β in the skin or serum of AI patients and healthy individuals by qRT‒PCR (f), ELISA (g), and IHC (h). Scale bar = 100 μm. AI patients (n = 7) and healthy individuals (n = 7) for panels (c–d). (i–k) IL-1β expression level in the skin or serum of normal mice and murine AI models using qRT‒PCR 7 (i), ELISA 7 (j) and IHC analysis (k). Scale bar = 100 μm. n = 8 mice per group for panels (i–k). *P < 0.05, **P < 0.01.
3.3. Infliximab downregulated IL-1β expression by inhibiting NLRP3
Inflammasomes control the bioactivity of the IL-1 family. Subsequently, the expression of inflammasome sensors (NLRP1, NLRP3, NLRC4, and AIM2) was investigated. The mRNA levels of NLRP1 and NLRP3 exhibited significant upregulation in AI lesions, and IFX treatment reduced the levels of these two genes in ex vivo skin explants from AI patients (Fig. 3a and b). A positive association between TNF and NLRP3 levels (R = 0.45, P < 0.05) (Fig. 3c) was analyzed, whereas no such relationship was observed between TNF and NLRP1 (R = 0.34, P > 0.05) (data from GSE154773) (Fig. S2). Therefore, we focused on the function of NLRP3. Similar to the mRNA results, the NLRP3 protein level was upregulated in AI patients and decreased in ex vivo skin explants of AI patients following IFX treatment (Fig. 3d and e). Moreover, an analogous elevation of NLRP3 expression was observed in skin lesions of the murine AI models compared to normal mice (Fig. 3f and g). These findings suggested that TNF was positively correlated with NLRP3 and that IFX could suppress the expression of NLRP3 in AI patient explants.
Fig. 3.
Infliximab treatment selectively downregulated NLRP3 expression levels. (a) qRT‒PCR technology was used to measure the level of inflammasome sensors in the skin lesions of AI patients compared to healthy individuals. (b) The level of inflammasomes in the skin lesions of AI patients with IFX (20 μg/mL) or PBS treatment using qRT‒PCR. (c) Correlation analysis of TNF and NLRP3 levels in the skin of AI patients and healthy subjects (data from GSE154773). n = 22 AI patients and n = 10 healthy individuals. (d) NLRP3 staining was performed in the skin of AI patients and healthy individuals via IF. Scale bar = 100 μm. (e) Immunostainings showed NLRP3 expression levels in the skin lesions of AI patients treated with IFX (20 μg/mL) or PBS. Scale bar = 100 μm. (f–g) NLRP3 expression level in the skin of normal mice and murine AI models by qRT‒PCR (f) and immunostainings (g). AI patients (n = 7) and healthy individuals (n = 7) for panels (a-b, d). n = 7 AI patients for panels (b, e). n = 8 mice per group for panels (f–g). Scale bar = 50 μm *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
3.4. Infliximab decreased NLRP3 and IL-1β levels by inhibiting NF-κB pathways in keratinocytes
Based on our results that anti-TNF-α treatment lowered the expression of NLRP3 and IL-1β, we performed a more comprehensive mechanistic study using HaCaT cells. The exposure of HaCaT cells to TNF-α only induced a substantial increase in NLRP3 expression rather than other inflammasomes, and the IL1B level was also enhanced (Fig. 4a). Moreover, Western blot and ELISA indicated that TNF-α stimulation also led to the upregulation of NLRP3 and IL-1β protein levels (Fig. 4b and Fig. S3a). This result indicated that TNF-α administration concurrently promoted the transcription and subsequent mature expression of IL-1β. Furthermore, GSEA from our sequencing data displayed a notable enrichment of genes correlated with the NF-κB signaling pathway and cytokine‒cytokine receptor interaction in the skin of patients with AI in the leading-edge subset (Fig. 4c). When HaCaT cells were exposed to TNF-α, the phosphorylation level of IκB was increased (Fig. 4d). In addition, NF-κB inhibitor (Bay 11–7082) treatment reduced the levels of NLRP3 and IL-1β after TNF-α stimulation (Fig. 4e and Fig. S3b), implying that TNF-α promoted IL-1β and NLRP3 expression by activating the NF-κB signaling pathway. To evaluate the effect of IFX on attenuating NF-κB and its downstream NLRP3/IL-1β pathways, HaCaT cells were pretreated with IFX and then stimulated with TNF-α. The results demonstrated that IFX abrogated TNF-dependent upregulation of p-IκB, NLRP3, and IL-1β (Fig. 4f and Fig. S3c).
Fig. 4.
TNF-α promoted NLRP3–IL-1β pathways by activating NF-κB signaling pathways in keratinocytes. (a) The level of inflammasomes and IL-1β expression in HaCaT cells with or without TNF-α (20 ng/mL) treatment using qRT‒PCR. n = 3 biologically independent samples. (b) Western blot was performed to semiquantify NLRP3 and IL-1β expression in HaCaT cells with or without TNF-α (20 ng/mL) treatment, and the grayscale analysis of NLRP3 and IL-1β was normalized to the unstimulated group. (c) GSEA plot showing the enrichment of genes upregulated in the NF-κB signaling pathway and cytokine‒cytokine receptor interaction. (d) P-IκB and IκB expression in HaCaT cells with or without TNF-α (20 ng/mL) treatment by Western blot, and the gray levels of P-IκB and IκB expression were normalized to those in the unstimulated group. (e) HaCaT cells were pre-treated with 10 μM BAY11-7082 for 1 h and TNF-α (20 ng/mL) was added into the media containing BAY11-7082 for another 24 h. NLRP3 and IL-1β expression was determined by Western blot. The grayscale analysis of NLRP3 and IL-1β expression was normalized to the unstimulated group. (f) HaCaT cells were incubated with 20 μg/mL IFX for 1 h and TNF-α (20 ng/mL) was added into the media containing IFX for another 24 h. P-IκB, IκB, NLRP3 and IL-1β expression was determined by Western blot. The grayscale analysis of p-IκB, IκB, NLRP3 and IL-1β expression was normalized to the unstimulated group. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
3.5. Infliximab alleviated the symptoms of murine AI models and downregulated NLRP3 and IL-1β expression
To evaluate the therapeutic effect of IFX in murine AI models, IFX was applied to mice twice after tamoxifen treatment (Fig. 5a). There was obvious improvement in the morphology of skin in murine AI models after IFX treatment (Fig. 5b). The curative effects of IFX were further confirmed by H&E staining, as indicated by decreased acanthosis and epidermal thickness (Fig. 5c). IHC and immunostaining results indicated that IFX could downregulate TNF-α, NLRP3 and IL-1β levels in the skin of murine AI models (Fig. 5d and e). Results in Figs. S4a–b indicated that serum levels of TNF-α and IL-1β were also downregulated in the murine AI models following IFX treatment. These data suggested that IFX application could alleviate AI symptoms by downregulating the NLRP3–IL-1β axis.
Fig. 5.
Infliximab treatment reduced TNF-α, IL-1β and NLRP3 expression levels in murine AI models. (a) Schematic diagram of murine AI models with or without IFX (5 mg/kg) treatment. (b) Gross appearance of skin lesions in murine AI models with or without IFX (5 mg/kg) treatment. (c) H&E staining of skin sections from normal mice, lesional skin of murine AI models, and lesional skin of murine AI models with IFX (5 mg/kg) therapy. Scale bar = 100 μm. (d) Representative images showing the protein levels of TNF-α and IL-1β in the skin of normal mice, murine AI models, and murine AI models with IFX (5 mg/kg) therapy via IHC analysis. Scale bar = 100 μm. (e) NLRP3 expression level in the skin of normal mice and murine AI models treated with IFX (5 mg/kg) or IgG by immunostainings. Scale bar = 50 μm. n = 8 mice per group for panels (a–e).
4. Discussion
The results obtained in our study indicated that both TNF-α and the NLRP3 inflammasome-related pathway were significantly enriched in the skin lesions of AI patients and murine AI models. Moreover, NLRP3 expression was normalized and IL-1β secretion was dramatically downregulated in AI patients explants after IFX therapy. These results were consistent with those of murine AI models. Mechanistically, IFX reduced the expression of NLRP3 and IL-1β by suppressing the NF-κB signaling pathway in keratinocytes.
TNF-α plays essential proinflammatory roles in the skin, and is produced by immune cells and keratinocytes [19]. Our clinicopathological examination indicated increased expression of TNF-α in AI skin lesions compared with heathy controls. In line with our investigation, previous studies reported that the serum expression level of TNF-α was increased in AI patients [20]. Indeed, GEO data analysis also confirmed that the TNF signaling pathway was enriched in patients with AI. Furthermore, we observed enhanced TNF-α levels in the skin lesions of our murine AI models. This rationale also underpinned our subsequent use of Ncstn conditional-knockout mice for in vivo investigations into the therapeutic mechanisms of IFX. Lowe et al. showed that TNF-α is a highly increased cytokine and that TNF-α-regulated molecules (IFNG, IL1B, and OSM) are also enriched in AI lesional skin using Ingenuity Pathway Analysis [21]. These results suggested TNF-α as a central hub in the pathogenesis of AI.
Thus far, targeted biologics, especially TNF-α inhibitors, have become a promising approach for AI [22]. IFX can neutralize both transmembrane receptor-bound and soluble TNF-α and deactivate its proinflammatory effect [23]. The symptoms of murine AI models were remarkably improved with IFX treatment by pathological examination. IL-1β is a highly prominent cytokine in AI lesional skin and is overexpressed even compared with psoriatic lesions [20]. IL-1β–induced transcriptomes in various cell types showed excessive upregulation of molecules causing immune cell infiltration and extracellular matrix degradation [24]. Furthermore, IL-1β was the most significantly reduced after IFX therapy in AI lesion skin using a different multiplex-based cytokine array, consistent with relevant reports by van der Zee [25]. Given the extensive and vital function of IL-1β, a first case report documented significant clinical improvement by an anti-IL-1β antibody (canakinumab) [26]. Collectively, the evidence above suggests that IFX treatment may effectively mitigate the clinical symptoms of AI by decreasing IL-1β expression. In addition, Lowe et al. reported that ADA treatment primarily attenuates B cell activation with minimal effects on the IL-1 signaling pathway [21]. Clinically, approximately half of treated patients fail to respond to ADA therapy. IL-1β expression tended to be elevated in nonresponders to ADA [21]; this may explain why IFX is more effective than ADA.
Biologically activated IL-1β is produced by the cleavage of pro-IL-1β, which is regulated by the inflammasome complex [27]. Our group and others have reported that the NLRP3 inflammasome is elevated in AI skin lesions [24,28]. In fact, IFX therapy may exert its therapeutic effects in AI patients and murine AI models in part by reducing the expression of NLRP3 and IL-1β in skin lesions, suggesting a tight relationship between inflammasome-related pathways and TNF-α. Similar to our results in AI, in psoriasis, ADA induced a remarkable decrease in NLRP3 and IL-1β [29]. Some research groups have shown that TNF-α or miR-155 can activate the NLRP3 inflammasome to induce psoriasis-related inflammatory responses [29,30]. In acne, the mechanism of NLRP3 activation depends on cellular K+ efflux and reactive oxygen species (ROS) [31]. To date, only one study has sought to investigate the cause of the increase in the NLRP3 inflammasome in AI. They reported that altered mRNA levels of multimeric NADPH oxidase (NOX) enzymes, which generate endogenous ROS, seem irrelevant to epidermis NLRP3 inflammasome activity in patients with AI [28]. It has been reported that NF-κB is an important transcription factor regulated by TNF-α [32]. The suppression of NF-κB viability could downregulate NLRP3 inflammasome expression in mouse mesangial cell strains [33]. In this study, TNF-α treatment induced NLPR3 inflammasome activation and increased IL-1β secretion, and one NF-κB inhibitor (Bay 11–7082) counteracted this effect, suggesting TNF-α-dependent NF-κB activation of the NLPR3 inflammasome in patients with AI.
There are still some limitations to our study. First, in addition to NLRP3, NLRP1 levels were also increased in AI skin tissue. However, TNF-α treatment of HaCaT cells did not induce NLRP1 expression, which might be due to the lack of a more suitable cell model. Second, many other cytokines that were reduced in AI skin explants after IFX treatment deserve further study. Despite these limitations, our report provides insights into the underlying mechanism of IFX treatment in the management of AI. It would be of interest to determine the benefit of therapeutic targeting of downstream targets such as the NLRP3 inflammasome relative to anti-TNF-α to help restore keratinocyte function in patients with AI.
5. Conclusion
Here, IFX was applied to keratinocytes, ex-vivo human AI skin explants and murine AI models. We found that both TNF-α and NLRP3 inflammasome-related pathways were enriched in skin lesions of AI patients and murine AI models. After IFX treatment, NLRP3 inflammasome-related pathway was effectively blocked and IL-1β level was normalized in ex-vivo AI skin explants and murine AI models. Mechanically, IFX suppressed NF-κB signaling pathway to lower the expression of NLRP3 and IL-1β in keratinocytes. These findings contribute to understand the action mechanism of IFX therapy.
Data and code availability statement
All data are available in the main text or the supplementary materials. The raw data for transcriptome sequencing are accessible at NCBI Gene Expression Omnibus (series record numbers: GSE148027 and GSE154773).
Ethics statement
This study was approved by the Medical Ethics Committee of the Chinese Academy of Medical Sciences Institute of Dermatology (2017-KY-015) and was performed according to the principles of the Declaration of Helsinki. Written informed consent was provided by all the individuals participating in the experiments. All animal experiments were approved by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Ethical Board of Nanjing University (IACUC-2204017).
CRediT authorship contribution statement
Yanyan He: Writing – original draft, Validation, Project administration, Investigation. Wenzhu Wang: Validation, Methodology, Formal analysis. Juan Jiang: Software, Data curation. Yuanxing Shen: Software, Data curation. Baoxi Wang: Conceptualization. Jiangning Chen: Writing – review & editing, Project administration, Conceptualization. Min Li: Writing – review & editing, Project administration, Funding acquisition. Haoxiang Xu: Writing – review & editing, Supervision, Project administration, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82073471, 82173432), the CAMS Innovation Fund for Medical Science (2017-I2M-1–017, 2021-I2M-1-001), Research program of geriatric health of Jiangsu Province (LK2021024).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e33146.
Contributor Information
Jiangning Chen, Email: jnchen@nju.edu.cn.
Min Li, Email: limin@pumcderm.cams.cn.
Haoxiang Xu, Email: xbpipi@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Saunte D.M.L., Jemec G.B.E. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318(20):2019–2032. doi: 10.1001/jama.2017.16691. [DOI] [PubMed] [Google Scholar]
- 2.Goldburg S.R., Strober B.E., Payette M.J. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J. Am. Acad. Dermatol. 2020;82(5):1045–1058. doi: 10.1016/j.jaad.2019.08.090. [DOI] [PubMed] [Google Scholar]
- 3.Matusiak L., Bieniek A., Szepietowski J.C. Increased serum tumour necrosis factor-alpha in hidradenitis suppurativa patients: is there a basis for treatment with anti-tumour necrosis factor-alpha agents? Acta Derm. Venereol. 2009;89(6):601–603. doi: 10.2340/00015555-0749. [DOI] [PubMed] [Google Scholar]
- 4.Hotz C., Boniotto M., Guguin A., Surenaud M., Jean-Louis F., Tisserand P., et al. Intrinsic defect in keratinocyte function leads to inflammation in hidradenitis suppurativa. J. Invest. Dermatol. 2016;136(9):1768–1780. doi: 10.1016/j.jid.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 5.Moran B., Sweeney C.M., Hughes R., Malara A., Kirthi S., Tobin A.M., et al. Hidradenitis suppurativa is characterized by dysregulation of the Th17:treg cell Axis, which is corrected by anti-TNF therapy. J. Invest. Dermatol. 2017;137(11):2389–2395. doi: 10.1016/j.jid.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 6.Savage K.T., Flood K.S., Porter M.L., Kimball A.B. TNF-alpha inhibitors in the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2019;10 doi: 10.1177/2040622319851640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balato A., Caiazzo G., Annunziata M.C., Marasca C., Scala E., Cacciapuoti S., et al. Anti-TNF-alpha therapy modulates mTORC1 signalling in hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2019;33(1):e43–e45. doi: 10.1111/jdv.15160. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y., Harvey B.P., Hong F., Ruzek M., Wang J., Murphy E.R., et al. Adalimumab induces a wound healing profile in patients with hidradenitis suppurativa by regulating macrophage differentiation and matrix metalloproteinase expression. J. Invest. Dermatol. 2021;141(11):2730–27340 e9. doi: 10.1016/j.jid.2021.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y., Hong F., Conlon D.M., Sidur L., Smith K.M., Fang Y., et al. Potential predictive biomarkers of adalimumab response in patients with hidradenitis suppurativa. Br. J. Dermatol. 2021;185(4):804–814. doi: 10.1111/bjd.20097. [DOI] [PubMed] [Google Scholar]
- 10.Shih T., Lee K., Grogan T., De D.R., Shi V.Y., Hsiao J.L. Infliximab in hidradenitis suppurativa: a systematic review and meta-analysis. Dermatol. Ther. 2022;35(9) doi: 10.1111/dth.15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ring H.C., Maul J.T., Yao Y., Wu J.J., Thyssen J.P., Thomsen S.F., et al. Drug survival of biologics in patients with hidradenitis suppurativa. JAMA Dermatol. 2022;158(2):184–188. doi: 10.1001/jamadermatol.2021.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frew J.W., Piguet V. Ex vivo models and interpretation of mechanistic studies in hidradenitis suppurativa. J. Invest. Dermatol. 2020;140(7):1323–1326. doi: 10.1016/j.jid.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Haferland I., Wallenwein C.M., Ickelsheimer T., Diehl S., Wacker M.G., Schiffmann S., et al. Mechanism of anti-inflammatory effects of rifampicin in an ex vivo culture system of hidradenitis suppurativa. Exp. Dermatol. 2022;31(7):1005–1013. doi: 10.1111/exd.14531. [DOI] [PubMed] [Google Scholar]
- 14.Vossen A., Ardon C.B., van der Zee H.H., Lubberts E., Prens E.P. The anti-inflammatory potency of biologics targeting tumour necrosis factor-alpha, interleukin (IL)-17A, IL-12/23 and CD20 in hidradenitis suppurativa: an ex vivo study. Br. J. Dermatol. 2019;181(2):314–323. doi: 10.1111/bjd.17641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Y., Xu H., Li C., Zhang X., Zhou P., Xiao X., et al. Nicastrin/miR-30a-3p/RAB31 Axis regulates keratinocyte differentiation by impairing EGFR signaling in familial acne inversa. J. Invest. Dermatol. 2019;139(1):124–134. doi: 10.1016/j.jid.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Lopetuso L.R., Petito V., Zinicola T., Graziani C., Gerardi V., Arena V., et al. Infliximab does not increase colonic cancer risk associated to murine chronic colitis. World J. Gastroenterol. 2016;22(44):9727–9733. doi: 10.3748/wjg.v22.i44.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J., Kang G., Lu H., de Marco A., Yuan H., Feng Z., et al. Novel bispecific nanobody mitigates experimental intestinal inflammation in mice by targeting TNF-alpha and IL-23p19 bioactivities. Clin. Transl. Med. 2024;14(3) doi: 10.1002/ctm2.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Revuz J. Hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. : JEADV. 2009;23(9):985–998. doi: 10.1111/j.1468-3083.2009.03356.x. [DOI] [PubMed] [Google Scholar]
- 19.Scala E., Cacciapuoti S., Garzorz-Stark N., Megna M., Marasca C., Seiringer P., et al. Hidradenitis suppurativa: where we are and where we are going. Cells. 2021;10(8) doi: 10.3390/cells10082094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Zee H.H., de Ruiter L., van den Broecke D.G., Dik W.A., Laman J.D., Prens E.P. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br. J. Dermatol. 2011;164(6):1292–1298. doi: 10.1111/j.1365-2133.2011.10254.x. [DOI] [PubMed] [Google Scholar]
- 21.Lowe M.M., Naik H.B., Clancy S., Pauli M., Smith K.M., Bi Y., et al. Immunopathogenesis of hidradenitis suppurativa and response to anti-TNF-alpha therapy. JCI Insight. 2020;5(19) doi: 10.1172/jci.insight.139932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wlodarek K., Ponikowska M., Matusiak L., Szepietowski J.C. Biologics for hidradenitis suppurativa: an update. Immunotherapy. 2019;11(1):45–59. doi: 10.2217/imt-2018-0090. [DOI] [PubMed] [Google Scholar]
- 23.Markota Cagalj A., Marinovic B., Bukvic Mokos Z. New and emerging targeted therapies for hidradenitis suppurativa. Int. J. Mol. Sci. 2022;23(7) doi: 10.3390/ijms23073753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witte-Handel E., Wolk K., Tsaousi A., Irmer M.L., Mossner R., Shomroni O., et al. The IL-1 pathway is hyperactive in hidradenitis suppurativa and contributes to skin infiltration and destruction. J. Invest. Dermatol. 2019;139(6):1294–1305. doi: 10.1016/j.jid.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 25.van der Zee H.H., Laman J.D., de Ruiter L., Dik W.A., Prens E.P. Adalimumab (antitumour necrosis factor-alpha) treatment of hidradenitis suppurativa ameliorates skin inflammation: an in situ and ex vivo study. Br. J. Dermatol. 2012;166(2):298–305. doi: 10.1111/j.1365-2133.2011.10698.x. [DOI] [PubMed] [Google Scholar]
- 26.Jaeger T., Andres C., Grosber M., Zirbs M., Hein R., Ring J., et al. Pyoderma gangrenosum and concomitant hidradenitis suppurativa--rapid response to canakinumab (anti-IL-1beta) Eur. J. Dermatol. 2013;23(3):408–410. doi: 10.1684/ejd.2013.2018. [DOI] [PubMed] [Google Scholar]
- 27.Xu J., Nunez G. The NLRP3 inflammasome: activation and regulation. Trends Biochem. Sci. 2023;48(4):331–344. doi: 10.1016/j.tibs.2022.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frings V.G., Sennefelder H., Presser D., Goebeler M., Schmidt M. Altered NOX expression does not seem to account for epidermal NLRP3 inflammasome activation in hidradenitis suppurativa. Br. J. Dermatol. 2019;181(2):391–392. doi: 10.1111/bjd.17647. [DOI] [PubMed] [Google Scholar]
- 29.Verma D., Fekri S.Z., Sigurdardottir G., Bivik Eding C., Sandin C., Enerback C. Enhanced inflammasome activity in patients with psoriasis promotes systemic inflammation. J. Invest. Dermatol. 2021;141(3):586–595 e5. doi: 10.1016/j.jid.2020.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Luo Q., Zeng J., Li W., Lin L., Zhou X., Tian X., et al. Silencing of miR-155 suppresses inflammatory responses in psoriasis through inflammasome NLRP3 regulation. Int. J. Mol. Med. 2018;42(2):1086–1095. doi: 10.3892/ijmm.2018.3677. [DOI] [PubMed] [Google Scholar]
- 31.Kistowska M., Gehrke S., Jankovic D., Kerl K., Fettelschoss A., Feldmeyer L., et al. IL-1beta drives inflammatory responses to propionibacterium acnes in vitro and in vivo. J. Invest. Dermatol. 2014;134(3):677–685. doi: 10.1038/jid.2013.438. [DOI] [PubMed] [Google Scholar]
- 32.Hayden M.S., Ghosh S. Regulation of NF-kappaB by TNF family cytokines. Semin. Immunol. 2014;26(3):253–266. doi: 10.1016/j.smim.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen J., Dai Z., Li Y., Zhu H., Zhao L. TLR9 regulates NLRP3 inflammasome activation via the NF-kB signaling pathway in diabetic nephropathy. Diabetol. Metab. Syndrome. 2022;14(1):26. doi: 10.1186/s13098-021-00780-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text or the supplementary materials. The raw data for transcriptome sequencing are accessible at NCBI Gene Expression Omnibus (series record numbers: GSE148027 and GSE154773).