Figure 1. Screening, Enrollment, Randomization, and Follow-up of Pediatric Participants.
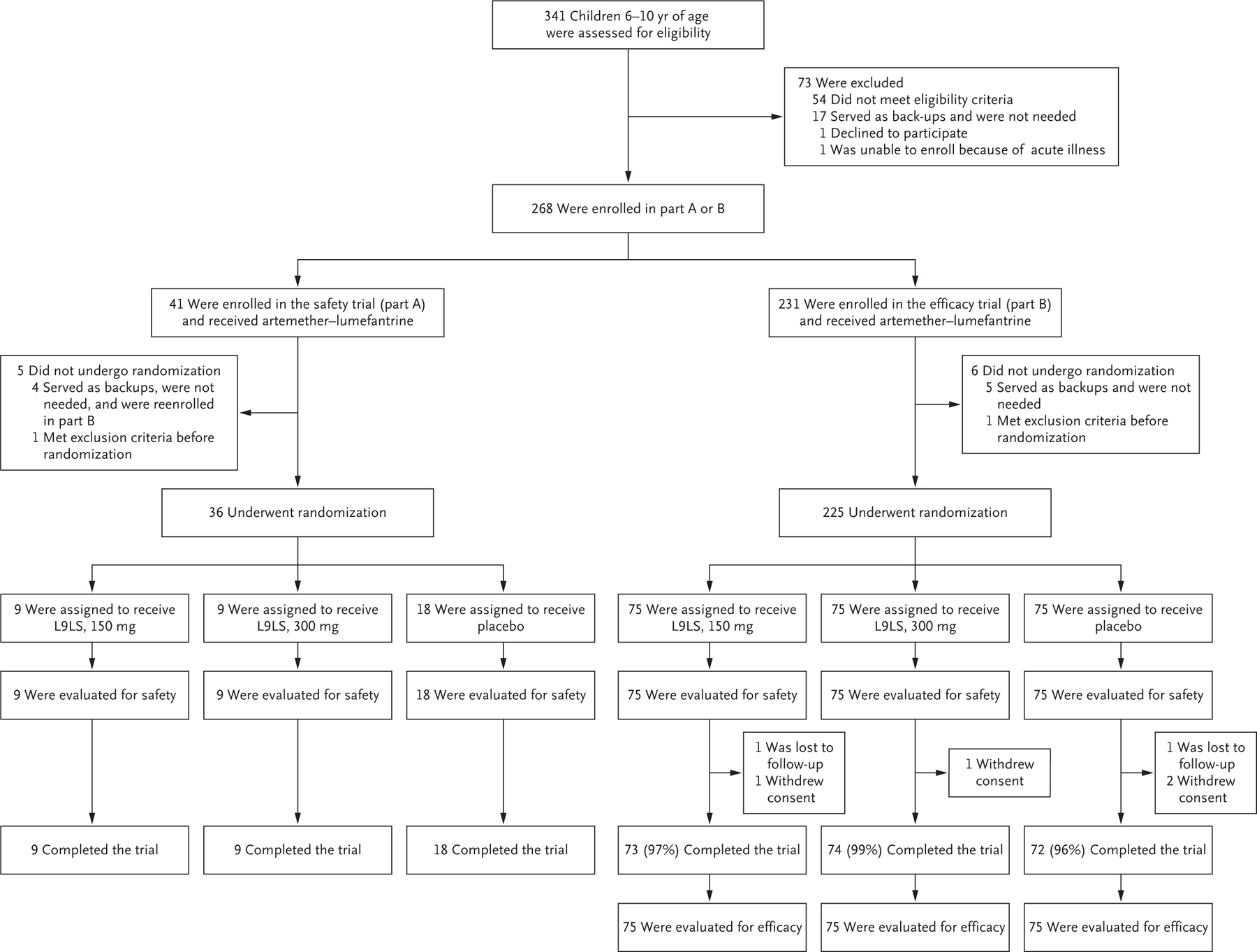
For pediatric participants, the trial was conducted in two parts. Part A was a double-blind, randomized, placebo-controlled, dose-escalation trial that was conducted before the malaria season to evaluate the safety and side-effect profile of the monoclonal antibody L9LS. Part B was a double-blind, randomized, placebo-controlled trial to assess the safety and efficacy of L9LS. A total of 268 participants were enrolled. Of the 41 participants who were initially enrolled in part A, 4 served as backups and were not needed but were reenrolled in part B. In part A, 36 participants underwent randomization between May 3 and May 17, 2022, and received a single subcutaneous injection of placebo or L9LS in one of two dose-escalation groups: 18 participants received placebo, 9 received 150 mg of L9LS, and 9 received 300 mg of L9LS. In part B, 225 participants underwent randomization between July 18 and August 15, 2022, and received a single subcutaneous injection of placebo or L9LS, with 75 participants in each group, before the peak of the malaria season. The final trial visits for part B occurred after the malaria season, on January 31, 2023. As prespecified in the protocol, the efficacy analysis was based on the modified intention-to-treat data set, which included all the participants who had undergone randomization and received L9LS or placebo, including those who withdrew or were lost to follow-up. In parts A and B of the trial, artemether–lumefantrine was given to all the participants as a standard, directly observed treatment course at enrollment, 7 to 12 days before the administration of L9LS or placebo, to clear any possible Plasmodium falciparum blood-stage infection.
