ABSTRACT
Morbidity and mortality caused by respiratory syncytial virus (RSV) in older adults and those with underlying health conditions can be potentially alleviated through vaccination. To assist vaccine policy decision-makers and payers, we estimated the annual economic burden of RSV-associated cardiorespiratory hospitalizations among insured US adults aged ≥18 y in the Merative MarketScan claims database from September through August of 2017–2018 and 2018–2019. Negative binomial regression models were used to estimate the number of RSV-associated cardiorespiratory hospitalizations using MarketScan-identified cardiorespiratory diagnosis codes in the presence or absence of RSV circulation per weekly laboratory test positivity percentages from the Centers for Disease Control and Prevention. This number was multiplied by mean cardiorespiratory hospitalization costs to estimate total costs for RSV-associated cardiorespiratory hospitalizations. Number and cost for International Classification of Diseases (ICD)-coded RSV hospitalizations were quantified from MarketScan. In 2017–2018 and 2018–2019, respectively, 18,515,878 and 16,462,120 adults with commercial or Medicare supplemental benefits were assessed. In 2017–2018, 301,248 cardiorespiratory hospitalizations were observed; 0.32% had RSV-specific ICD codes, costing $44,916,324, and 5.52% were RSV-associated cardiorespiratory hospitalizations, costing $734,078,602 (95% CI: $460,826,580–$1,103,358,799). In 2018–2019, 215,525 cardiorespiratory hospitalizations were observed; 0.34% had RSV-specific ICD codes, costing $33,053,105, and 3.14% were RSV-associated cardiorespiratory hospitalizations, costing $287,549,472 (95% CI: $173,377,778–$421,884,259). RSV contributes to substantial economic burden of cardiorespiratory hospitalizations among US adults. Modeling excess risk using viral positivity data provides a comprehensive estimation of RSV hospitalization burden and associated costs, compared with relying on ICD diagnosis codes alone.
KEYWORDS: RSV, respiratory, cardiorespiratory hospitalizations, economic burden, excess risk modeling
Introduction
Respiratory syncytial virus (RSV) is a highly infectious seasonal respiratory virus primarily affecting infants and young children, but certain adult populations (ie, immunocompromised, or with high-risk conditions such as chronic heart or lung disease) are also at risk of severe RSV infection. The annual incidence of RSV infection is 4–10% among adults who have the greatest risk for severe outcomes, such as hospitalization.1,2 Between 60,000 and 160,000 older adults are hospitalized and between 6000 and 10,000 die from RSV infection annually in the United States (US).3 However, the true burden of RSV infections among older adults and individuals with chronic high-risk conditions may be underestimated due to the underdetection of RSV.4
While it is well understood that influenza is associated with an increased risk of cardiovascular outcomes, such as myocardial infarction and stroke, emerging evidence extends these findings to other respiratory viral infections, including RSV.5–8 A retrospective cohort study in Canada observed that up to 22% of adults hospitalized for RSV infection experienced cardiovascular complications, including heart failure (HF) (14%), arrhythmia (8%), stroke (2%), and myocardial infarction (1%).8 RSV infection also contributes to adverse respiratory outcomes. A retrospective 2-year chart review of adults with laboratory-confirmed RSV reported a high incidence of respiratory complications among adults who were aged ≤65 y (50%), aged >65 y (54.7%), immunocompromised (62.5%), and diagnosed with chronic obstructive pulmonary disease (COPD) (87.1%).5 Thus, it is important to understand the downstream impact of RSV infections when evaluating the overall clinical burden of RSV.
In addition to clinical morbidity, hospitalizations associated with RSV infections burden the health care system economically. In a cost study nested within a large prospective study of adults aged ≥18 y with RSV-associated hospitalizations, annual RSV-associated hospitalization costs were estimated to be $1.3 billion for US adults, primarily driven by a greater incidence of RSV infections among older adults.9 Several US studies have varied widely in their estimates of RSV-associated health care costs and relied only on RSV-specific diagnosis codes to ascertain RSV cases.10–14 However, due to a lack of routine testing for RSV, relying on International Classification of Diseases (ICD) diagnosis codes may underestimate the complete burden of RSV.15–17 For instance, a recent modeling study estimated that among older adults with RSV-associated hospitalizations, <5% of these hospitalizations listed a diagnosis code for RSV,17 and a review article reported that about 80% of hospitals performed RSV testing in less than 25% of hospitalizations related to lower respiratory tract infection.16
Alternatively, clinical and economic burden can be estimated more completely via excess risk modeling, which estimates outcomes associated with the infectious disease of interest, a common approach used in surveillance epidemiology.18 In a retrospective cohort study using excess risk modeling on a US nationwide sample of cardiorespiratory hospitalizations from 1997 to 2009, Matias and colleagues estimated that, annually, approximately 200,000 cardiorespiratory hospitalizations were associated with RSV.18 More recently, Bosco and colleagues conducted a retrospective cohort study of nearly 3 million US long-term care (LTC) residents from 2011 to 2017 and estimated the burden of RSV-attributable cardiorespiratory hospitalizations via excess risk modeling.19 The authors reported a total of approximately 6200 RSV-attributable cardiorespiratory hospitalizations among LTC residents, with a cost of more than $51 million over the entire study period. However, to further the understanding of the impact of RSV, it is also important to understand the economic burden of RSV-associated hospitalizations among a broader sample of insured community-dwelling adults in the US.
Our primary objective was to estimate the annual economic burden of RSV infection on cardiorespiratory hospitalizations among insured US adults, using an excess risk model, and to describe differences in burden estimates when relying on International Classification of Diseases, 10th revision (ICD-10), diagnosis codes alone. As a secondary objective, we aimed to describe health care resource utilization (HCRU) characteristics of ICD-coded RSV hospitalizations among these insured adults. Another secondary objective was to estimate the annual economic burden of RSV infection on cardiorespiratory hospitalizations among a subgroup of high-risk adults. Currently, two protein-based vaccines are approved in the US for the prevention of RSV-associated lower respiratory tract disease in adults aged ≥60 y,20 and an mRNA-based RSV vaccine showing promising safety and efficacy results is in the late stages of clinical development.21 Therefore, the goal of this study was to provide vaccine policy decision-makers and payers with a more complete understanding of the economic burden of RSV infections on cardiorespiratory hospitalizations among adults, particularly those at high risk for severe RSV.
Materials and methods
Data sources
We identified our study population from closed administrative claims in the following Merative MarketScan databases: Commercial Claims and Encounters (CCAE), Medicare Supplemental and Coordination of Benefits (MDCR), and Medicaid Multi-State (MDCD).22 In addition to individual-level enrollment and demographic data, the MarketScan databases include inpatient and outpatient medical claims and outpatient prescription drug claims. US geographic region data are limited to individuals in the CCAE and MDCR databases. Race/ethnicity data are also available but limited to individuals in the MDCD database.
For use in modeling, we acquired weekly laboratory percentage positivity for RSV from the Centers for Disease Control and Prevention’s (CDC) National Respiratory and Enteric Virus Surveillance System (NREVSS), a passive surveillance network that collects laboratory data from participating US clinical and public health laboratories.23
Study design and population
We conducted a retrospective cohort study of adults who were insured under a commercial, Medicare supplemental, or Medicaid payer in the MarketScan database. Separate cohorts were assessed for 2017–2018 and 2018–2019, with each study year spanning from September through August. Patients entered the cohort in September through May of the study year, after being continuously enrolled throughout the baseline period. The baseline period was 242 d (for 2017–2018) or 365 d (for 2018–2019) before the start of the study year, given the MarketScan data date range (September 30, 2016, to September 30, 2021) available. Patients were excluded from the cohort if their age was missing or <18 y on the date of cohort entry, if they switched enrollment from a commercial to a Medicare supplemental payer in the baseline period, or if they did not have commercial, Medicare supplemental, or Medicaid enrollment in the baseline period, excluding dual eligibility for Medicare and Medicaid benefits.
All analyses were conducted by payer type categorized as commercial or Medicare supplemental (commercial/Medicare supplemental) and, separately, as Medicaid. Additional subgroup analyses, stratified by payer type, were conducted among adults defined as being at high risk for severe RSV, based on having ≥1 of the following conditions in the baseline period: asthma, COPD, HF, coronary artery disease (CAD), diabetes mellitus (DM), advanced liver disease, chronic kidney disease/end-stage renal disease (CKD/ESRD), or immunocompromised status.3,24–27 Code algorithms for high-risk conditions and immunocompromised status can be found in Tables S1 and S2. We present study findings for the commercial/Medicare supplemental population only in the main text of this paper. Additional details for the commercial/supplemental population and study findings for the Medicaid population are presented in supplemental material.
Age, sex, race/ethnicity (Medicaid only) and US geographic region (commercial/Medicare supplemental only) were assessed on the cohort entry date. In addition to high-risk conditions and immunocompromised status, the combined comorbidity index (CCI) and the claims-based frailty index (CFI) were assessed over the baseline period.28–31 Frailty status was reported as robust (CFI <0.25), prefrail or midfrailty (CFI 0.25 to <0.35), and moderate-to-severe frailty (CFI 0.25 to <0.35).32 Code algorithms for the CCI and CFI can be found in Tables S3 and S4.
Measurement of ICD-coded hospitalizations
We identified cardiorespiratory hospitalizations from MarketScan claims, based on an International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis code (A37.91, B34×, B97.4, I20×–I22×, I27×, I47×–I50×, J00×–J06×, J09×–J18×, J20×–J22×, J30×–J39×, J41×–J45×, J47×, J80×–J82×, J84×, J96×, J99×, P27×, Q33.4, R05×, R06×, R50×) in any position. Patients were followed for their first ICD-coded cardiorespiratory hospitalization (henceforth referred to as “cardiorespiratory hospitalization”) in the study year, given that <1% of patients had multiple cardiorespiratory hospitalizations in a study year. Additionally, we identified hospitalizations from MarketScan with an ICD-10-CM diagnosis code for RSV specifically (B97.4, J12.1, J20.5, J21.0), in any position. Patients were followed for their first RSV hospitalization in a study year. For these ICD-coded RSV hospitalizations, the following HCRU characteristics were described: length of stay (LOS), intubation, intermittent mandatory ventilation, noninvasive ventilation, ventilation procedure without invasive specification, supplemental oxygen, extracorporeal membrane oxygenation, and hospitalization discharge status. Code algorithms for described HCRU characteristics can be found in Table S5.
Statistical analysis
We estimated the number of RSV-associated cardiorespiratory hospitalizations in each study year via zero-truncated negative binomial regression models previously described by Bosco and colleagues and Matias and associates and detailed in the flumodelr R package developed by Bosco and coworkers.18,33,34 For model input, weekly aggregated counts of cardiorespiratory hospitalizations and weekly aggregated total person-time from MarketScan claims were combined with weekly RSV laboratory positivity percentages from the NREVSS laboratory surveillance data into a single analytic dataset. Patients were censored on disenrollment or the end of the study year. Candidate models were fit using different linear combinations of the following parameters:
Week number (continuous) with sine and/or cosine transformations for quarterly periodicity trends
Week number (continuous) with quadratic and/or cubic transformations to model nonlinear, aperiodic time trends
Indicator for RSV activity, with and without a 2-week lag accounting for the delay between viral testing after presentation with symptoms and occurrence of RSV-associated cardiorespiratory hospitalizations
Final model selection for the overall samples by payer type was chosen using the lowest Akaike information criterion (AIC) value. Model parameters from these final models were applied to the high-risk subgroup for each payer type.
The final model form included a population offset term for the natural log-transformed number of person-weeks at risk (α), quadratic and cubic polynomial terms for number of weeks from the first observed time point to capture nonlinear, aperiodic time trends (β1, β2, and β3), a Fourier series term for quarterly periodicity trends (β4 and β5), and an indicator for weekly percentage positive RSV specimens that varied with and without a 2-week lag (β6). A 2-week lag term was adopted given that our study relied on RSV laboratory positivity data from the CDC, which defines cases as those with a positive RSV test within 14 d of a hospitalization.35 We used quarterly sine and cosine terms to separately model each study year. In the model formula below, E(Yi) represents the expected number of cardiorespiratory hospitalizations identified in MarketScan claims during a given week (ti).
The final model was fit to estimate the weekly number of cardiorespiratory hospitalizations in the presence of RSV (ie, β6[RSV] viral term). Then, a different model was fit to estimate cardiorespiratory hospitalizations in the absence of RSV, by setting the viral term to 0. The difference between weekly estimates from the two models represented the weekly number of RSV-associated cardiorespiratory hospitalizations and was summed for each study year to estimate the total number of RSV-associated cardiorespiratory hospitalizations for that study year. Given the derived nature of RSV-associated cardiorespiratory hospitalization estimates, 95% confidence intervals (CI) were estimated using a residual block bootstrapping approach, which accounts for autocorrelation of time-series data (Method Supplement). Analytic dataset preparation and modeling were conducted using the Aetion® Substantiate platform, version 4.65 and R version 4.2.1.36,37
If the best-fitting model resulted in a negative estimate for the number of RSV-associated cardiorespiratory hospitalizations, a 95% CI was calculated around the point estimate, and if the 95% CI included 0, findings were interpreted as null. Additionally, models were not fit for a given subgroup and year if fewer than 1500 cardiorespiratory hospitalizations were identified in MarketScan claims, due to insufficient background rates to produce stable model estimates.
Rate, cost, and LOS calculations
We calculated the rate of ICD-coded RSV hospitalizations as the number of ICD-coded RSV hospitalizations identified in MarketScan divided by total person-years (PYs) of follow-up for the study year. Corresponding 95% CIs were calculated using a robust variance estimator from a Poisson regression model.
We estimated the rate of RSV-associated cardiorespiratory hospitalizations by dividing the number of RSV-associated cardiorespiratory hospitalizations in a study year, estimated from the negative binomial regression model, by the total PYs of follow-up in the study year. Corresponding 95% CI was calculated by dividing the lower and upper confidence limits for the number of RSV-associated cardiorespiratory hospitalizations by the total PYs of follow-up in the study year. All rates and 95% CIs were presented as the number of events per 100,000 PYs.
We calculated the total cost for ICD-coded RSV hospitalizations by summing the cost for all ICD-coded RSV hospitalizations identified in MarketScan in the study year. We estimated the outcome of the total cost for RSV-associated cardiorespiratory hospitalizations by multiplying the mean cost for cardiorespiratory hospitalizations identified in MarketScan by the number of RSV-associated cardiorespiratory hospitalizations, estimated from the negative binomial regression model, in the study year. Corresponding 95% CIs were calculated by multiplying the lower and upper confidence limits for the number of RSV-associated cardiorespiratory hospitalizations by the mean cost for cardiorespiratory hospitalizations in the study year. The mean of cardiorespiratory hospitalization costs was used to estimate total RSV-associated cardiorespiratory hospitalization costs in order to directly compare our study findings with prior estimates of excess RSV- and influenza-associated hospitalization costs.19,38 Costs were converted to 2022 inflation-adjusted US dollars using the medical care services component of the Consumer Price Index from the US Bureau of Labor Statistics.39 Total costs were reported as the sum of the two cost portions paid by the payer and by the patient on hospitalization claims. Payer cost was summed from the coordination of benefits and the net payment by the payer, excluding patient out-of-pocket costs and coordination of benefits. Patient costs were summed from deductibles, coinsurance, and copayments.
Sensitivity analysis
We conducted a sensitivity analysis on the overall study population to assess potential bias in estimates of the number of RSV-associated cardiorespiratory hospitalizations due to differential censoring, using inverse probability of censoring weighting (IPCW).40,41 By assigning a greater weight to noncensored individuals who more closely resembled censored ones, the IPCW analysis reweighted the study population to represent a pseudopopulation in which no observation was censored. Age, sex, payer type, CCI score (categorized as 0, 1–2, and ≥3), frailty status, and high-risk status were included in a logistic regression model to calculate censoring weights. Then, the weighted average of the censoring weights was used in the zero-truncated negative binomial regression model from the primary analysis to regenerate estimates of the total number of RSV-associated cardiorespiratory hospitalizations. A block bootstrapping approach was used to generate 95% CIs (Method Supplement).
Results
Study population characteristics
Across all payer types 22,617,080 and 19,794,099 individuals met cohort criteria in study years 2017–2018 and 2018–2019, respectively (Table S6). The study population consisted of 18,515,878 and 16,462,120 adults with commercial/Medicare supplemental enrollment in 2017–2018 and 2018–2019, respectively (Table 1). For commercial/Medicare supplemental patients, the mean age was 44.25 y and 44.02 y, and 52.7% and 52.3% of patients were female, in 2017–2018 and 2018–2019, respectively. The high-risk subgroup represented 13.5% and 16.1% of commercial/Medicare supplemental adults overall in 2017–2018 and 2018–2019, respectively (Table 2). Compared with commercial/Medicare supplemental adults overall, high-risk adults were older, had a similar sex distribution, and were more likely to be enrolled in Medicare supplemental benefits, have comorbidities, be immunocompromised, and be frail.
Table 1.
Patient characteristics for study population, commercial/Medicare supplemental adults overall.
| Patient Characteristics | 2017–2018 N = 18,515,878 |
2018–2019 N = 16,462,120 |
|---|---|---|
| Age (years)a | ||
| Mean (SD) | 44.25 (15.93) | 44.02 (15.50) |
| Median [IQR] | 45.00 [31.00, 56.00] | 45.00 [31.00, 56.00] |
| Sex, n (%)a | ||
| Female | 9,756,608 (52.7%) | 8,606,204 (52.3%) |
| Male | 8,759,270 (47.3%) | 7,855,916 (47.7%) |
| US geographic region, n (%)a,b | ||
| Northeast | 3,322,102 (17.9%) | 3,142,764 (19.1%) |
| North Central | 3,848,178 (20.8%) | 3,555,595 (21.6%) |
| South | 8,425,560 (45.5%) | 7,244,239 (44.0%) |
| West | 2,879,499 (15.6%) | 2,471,811 (15.0%) |
| Unknown | 40,539 (0.2%) | 47,711 (0.3%) |
| Payer type, n (%)c | ||
| Commercial | 17,177,645 (92.8%) | 15,527,164 (94.3%) |
| Medicare Supplemental | 1,338,233 (7.2%) | 934,956 (5.7%) |
| Conditions at high risk of severe RSV, n (%)c | ||
| Chronic obstructive pulmonary disease | 163,210 (0.9%) | 152,964 (0.9%) |
| Asthma | 340,201 (1.8%) | 502,778 (3.1%) |
| Diabetes mellitus | 1,233,399 (6.7%) | 1,227,110 (7.5%) |
| Congestive heart failure | 130,899 (0.7%) | 127,058 (0.8%) |
| Advanced liver disease | 190,091 (1.0%) | 261,610 (1.6%) |
| Chronic kidney disease or end-stage renal disease | 230,554 (1.2%) | 234,860 (1.4%) |
| Coronary artery disease | 387,386 (2.1%) | 401,744 (2.4%) |
| Immunocompromised status, n (%)d | 561,180 (3.0%) | 539,296 (3.3%) |
| Combined comorbidity indexc,e | ||
| Mean (SD) | 0.25 (1.05) | 0.32 (1.14) |
| Median [IQR] | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] |
| Claims-based frailty index, n (%)c,f | ||
| Robust (<0.25) | 17,151,567 (92.6%) | 15,096,367 (91.7%) |
| Prefrail or mild frailty (0.25 to <0.35) | 1,353,507 (7.3%) | 1,355,537 (8.2%) |
| Moderate to severe frailty (≥0.35) | 10,804 (0.1%) | 10,216 (0.1%) |
| Length of follow-up (days), mean (SD)g | 284.95 (114.22) | 257.17 (113.74) |
Abbreviations: ICD = International Classification of Diseases; IQR = interquartile range; RSV = respiratory syncytial virus; SD = standard deviation; US = United States.
aAssessed on cohort entry date.
bUS geographic region data are only available in MarketScan’s Commercial Claims and Encounters and Medicare Supplemental and Coordination of Benefits databases and is not available in the Multi-State Medicaid database.
cAssessed in the 242 d and 365 d before cohort entry in 2017–2018 and 2018–2019, respectively, corresponding to the baseline period for each study year.
dAssessed using varying lookback periods before cohort entry, based on Polinski et al.27
gLength of follow-up is presented in days. Patients were followed from cohort entry until their first ICD-coded cardiorespiratory hospitalization, disenrollment, or the end of study year.
Table 2.
Patient characteristics for study population, commercial/Medicare supplemental adults at high risk for severe RSV.
| Patient Characteristics | 2017–2018 N = 2,493,686 |
2018–2019 N = 2,649,397 |
|---|---|---|
| Age (years)a | ||
| Mean (SD) | 57.00 (15.05) | 54.88 (14.67) |
| Median [IQR] | 58.00 [48.00, 64.00] | 56.00 [47.00, 63.00] |
| Sex, n (%)a | ||
| Female | 1,291,014 (51.8%) | 1,372,301 (51.8%) |
| Male | 1,202,672 (48.2%) | 1,277,096 (48.2%) |
| US geographic region, n (%)a,b | ||
| Northeast | 490,658 (19.7%) | 566,705 (21.4%) |
| North Central | 550,516 (22.1%) | 589,717 (22.3%) |
| South | 1,149,262 (46.1%) | 1,167,223 (44.1%) |
| West | 299,338 (12.0%) | 320,622 (12.1%) |
| Unknown | 3912 (0.2%) | 5130 (0.2%) |
| Payer type, n (%)c | ||
| Commercial | 1,897,421 (76.1%) | 2,200,488 (83.1%) |
| Medicare Supplemental | 596,265 (23.9%) | 448,909 (16.9%) |
| Conditions at high risk of severe RSV, n (%)c | ||
| Chronic obstructive pulmonary disease | 163,210 (6.5%) | 152,964 (5.8%) |
| Asthma | 340,201 (13.6%) | 502,778 (19.0%) |
| Diabetes mellitus | 1,233,399 (49.5%) | 1,227,110 (46.3%) |
| Congestive heart failure | 130,899 (5.2%) | 127,058 (4.8%) |
| Advanced liver disease | 190,091 (7.6%) | 261,610 (9.9%) |
| Chronic kidney disease or end-stage renal disease | 230,554 (9.2%) | 234,860 (8.9%) |
| Coronary artery disease | 387,386 (15.5%) | 401,744 (15.2%) |
| Immunocompromised status, n (%)d | 561,180 (22.5%) | 539,296 (20.4%) |
| Combined comorbidity indexc,e | ||
| Mean (SD) | 1.32 (2.11) | 1.37 (2.07) |
| Median [IQR] | 1.00 [0.00, 2.00] | 1.00 [0.00, 2.00] |
| Claims-based frailty index, n (%)c,f | ||
| Robust (<0.25) | 1,704,850 (68.4%) | 1,861,011 (70.2%) |
| Prefrail or mild frailty (0.25 to <0.35) | 779,170 (31.2%) | 779,343 (29.4%) |
| Moderate to severe frailty (≥0.35) | 9666 (0.4%) | 9043 (0.3%) |
| Length of follow-up (days), mean (SD)g | 269.71 (121.02) | 257.18 (117.07) |
Abbreviations: ICD = International Classification of Diseases; IQR = interquartile range; RSV = respiratory syncytial virus; SD = standard deviation; US = United States.
aAssessed on cohort entry date.
bUS geographic region data is only available in MarketScan’s Commercial Claims and Encounters and Medicare Supplemental and Coordination of Benefits databases and is not available in the Multi-State Medicaid database.
cAssessed in the 242 d and 365 d before cohort entry in 2017–2018 and 2018–2019, respectively, corresponding to the baseline period for each study year.
dAssessed using varying lookback periods before cohort entry, based on Polinski et al.27
gLength of follow-up is presented in days. Patients were followed from cohort entry until their first ICD-coded cardiorespiratory hospitalization, disenrollment, or the end of study year.
The study population consisted of 4,101,202 and 3,331,979 adults with Medicaid enrollment in 2017–2018 and 2018–2019, respectively (Table S6). Compared with commercial/Medicare supplemental patients, Medicaid patients were younger and were more likely to be female, have COPD, asthma, HF, or advanced liver disease, be immunocompromised, and be prefrail to mildly frail (Table S7). High-risk adults were sicker and frailer in the Medicaid population, compared with commercial/Medicare supplemental (Table S8).
ICD-coded RSV hospitalizations and RSV-associated cardiorespiratory hospitalizations
In 2017–2018, among commercial/Medicare supplemental adults, 301,248 cardiorespiratory hospitalizations were identified, with 52.4% occurring among high-risk adults (Table S9). Among all cardiorespiratory hospitalizations, while only 0.32% had an RSV-specific ICD diagnosis code, 5.52% were estimated to be associated with RSV infection, based on excess risk modeling. Among all cardiorespiratory hospitalizations for high-risk adults, the percentage of model-estimated RSV-associated cardiorespiratory hospitalizations was even higher at 6.41% (Figure 1a). Similarly, in 2018–2019, 215,525 ICD-coded cardiorespiratory hospitalizations were identified, with 56.4% occurring among high-risk adults (Table S9). Among all cardiorespiratory hospitalizations, while 0.34% had an RSV-specific ICD diagnosis code, 3.14% were estimated to be associated with RSV infection, based on excess risk modeling. Among all cardiorespiratory hospitalizations for high-risk adults, the percentage of model-estimated RSV-associated cardiorespiratory hospitalizations was even higher at 5.89% (Figure 1b).
Figure 1.
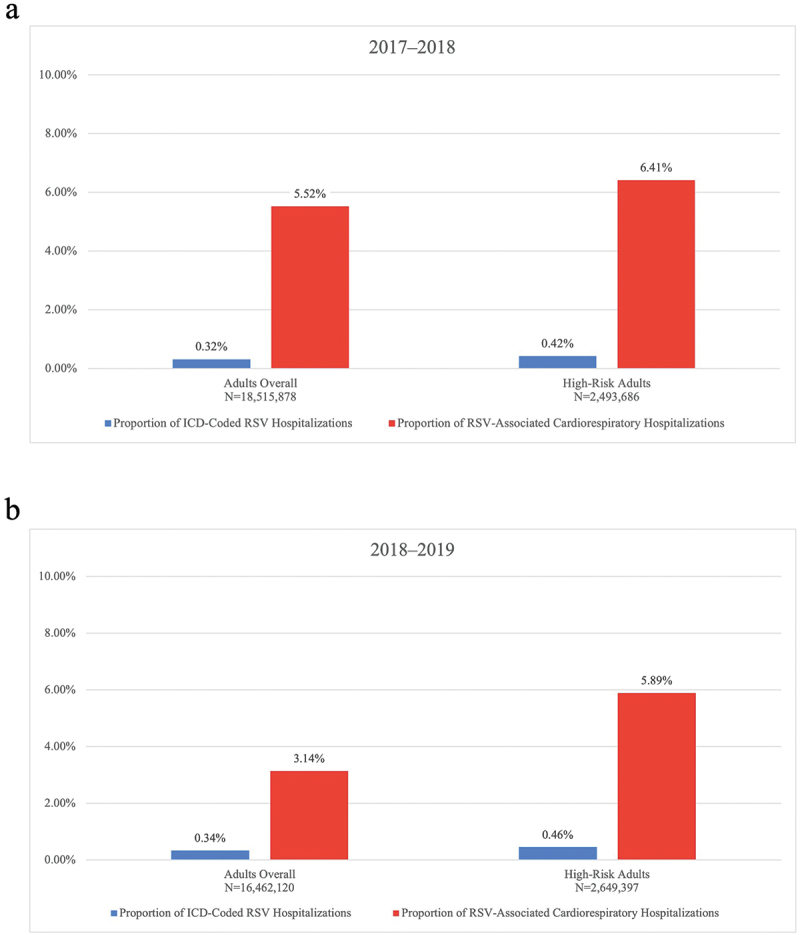
Percentage of ICD-coded RSV hospitalizations and RSV-associated cardiorespiratory hospitalizations, commercial/Medicare supplemental adults, (a) 2017–2018, (b) 2018–2019. Percentages are calculated using a denominator of the total ICD-coded cardiorespiratory hospitalizations. ICD = International Classification of Diseases; RSV = respiratory syncytial virus.
Findings were mostly similar in the Medicaid population. However, in 2017–2018, the percentage of RSV-associated cardiorespiratory hospitalizations was approximately two-fold larger (12.82% vs 5.52%), compared with commercial/Medicare supplemental adults. Additional data for hospitalization counts, percentages, and rates in the commercial/Medicare supplemental and Medicaid populations are reported in Tables S9 and S10.
HCRU among ICD-coded RSV hospitalizations
In 2017–2018, the mean LOS for ICD-coded RSV hospitalizations was 8.41 d in 2017–2018 and 7.78 d in 2018–2019 (Table 3). Of these hospitalizations, 6.9%–8.7% included intubation, 19.8%–20.6% included ventilation, and 51.9%–55.7% included supplemental oxygen across study years. Almost two-thirds of patients were discharged to home self-care, 13.9%–14.7% were discharged home under care, and 9.8%–10.6% were transferred to a skilled nursing facility across study years.
Table 3.
Health care resource utilization characteristics of ICD-coded RSV hospitalizations, adults overall.
| Patient Characteristics | 2017–2018 N = 24,362,080 |
2018–2019 N = 21,359,058 |
|---|---|---|
| Total ICD-coded RSV hospitalizations, n | 1843 | 1597 |
| Length of stay (days)a | ||
| Mean (SD) | 8.41 (11.31) | 7.78 (8.64) |
| Median [IQR] | 6.00 [4.00, 9.00] | 6.00 [4.00, 9.00] |
| Treatment, n (%) | ||
| Intubation | 161 (8.7%) | 110 (6.9%) |
| Intermittent mandatory ventilation | 47 (2.6%) | 29 (1.8%) |
| Non-invasive ventilation | 163 (8.8%) | 165 (10.3%) |
| Ventilation procedure without invasive specification | 170 (9.2%) | 123 (7.7%) |
| Supplemental oxygen | 957 (51.9%) | 890 (55.7%) |
| Extracorporeal membrane oxygenation | 0 (0.0%) | 5 (0.3%) |
| Discharge status, n (%) | ||
| Discharged to home self-care | 1175 (63.8%) | 976 (61.1%) |
| Discharged home under care | 256 (13.9%) | 235 (14.7%) |
| Transferred to skilled nursing facility | 180 (9.8%) | 170 (10.6%) |
| Transferred to inpatient rehabilitation facility | 18 (1.0%) | 12 (0.8%) |
| Transferred to short term hospital | 43 (2.3%) | 25 (1.6%) |
| Transferred to long term care hospital | 31 (1.7%) | 21 (1.3%) |
| Left against medical advice | 9 (0.5%) | 14 (0.9%) |
| Discharged to home hospice | 16 (0.9%) | 13 (0.8%) |
| Discharged to hospice facility | 16 (0.9%) | 19 (1.2%) |
| Other | 79 (4.3%) | 83 (5.2%) |
Abbreviations: ICD = International Classification of Diseases; IQR = interquartile range; RSV = respiratory syncytial virus; SD = standard deviation.
aLength of stay was calculated as the count of days from the start through the end of each hospitalization claim and is presented in days.
Costs for ICD-coded RSV hospitalizations and RSV-associated cardiorespiratory hospitalizations
In 2017–2018, among commercial/Medicare supplemental adults (N = 18,515,878), total costs for ICD-coded RSV hospitalizations and RSV-associated cardiorespiratory hospitalizations, respectively, were $44,916,324 and $734,078,602 (95% CI: $460,826,580–$1,103,358,799), with total costs of $34,971,264 and $451,444,885 (95% CI: $237,166,483–$609,193,987) among high-risk adults (N = 2,493,686) (Figure 2a).
Figure 2.
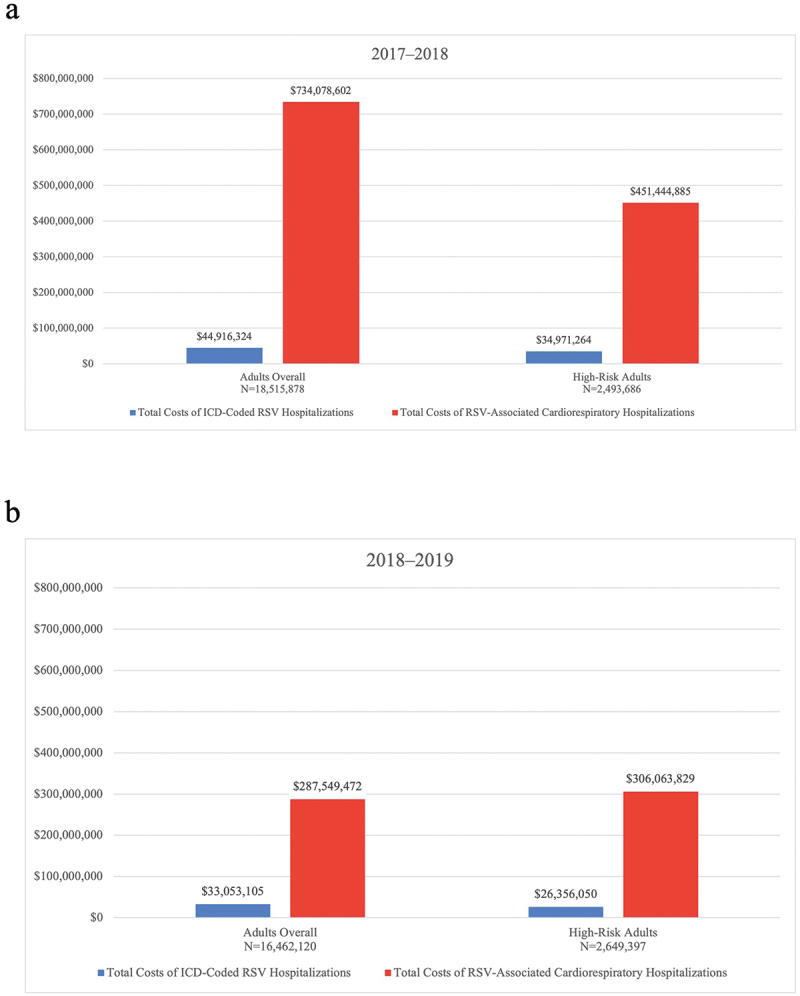
Total costs for ICD-coded RSV hospitalizations and RSV-associated cardiorespiratory hospitalizations, commercial/Medicare supplemental adults, (a) 2017–2018, (b) 2018–2019. Costs are presented in US dollars and are adjusted for 2022 inflation using the medical care services component of the Consumer Price Index from the US Bureau of Labor Statistics. ICD = International Classification of Diseases; RSV = respiratory syncytial virus.
In 2018–2019, among commercial/Medicare supplemental adults (N = 16,462,120), total costs for ICD-coded RSV hospitalizations and RSV-associated cardiorespiratory hospitalizations, respectively, were $33,053,105 and $287,549,472 (95% CI: $173,377,778–$421,884,259), with total costs of $26,356,050 and $306,063,829 (95% CI: $195,013,315–$407,702,997) among high-risk adults (N = 2,649,397) (Figure 2b). More details on cost findings in the commercial/Medicare supplemental population can be found in Table S11. Total costs among adults enrolled in Medicaid are provided in Table S12.
Sensitivity analysis
Compared with the primary analysis, the sensitivity analysis accounting for informative censoring via IPCW resulted in consistent, but slightly lower, estimates of the number of RSV-associated cardiorespiratory hospitalizations of 49,869 (95% CI: 38,370–61,127) and 25,375 (95% CI: 17,580–35,129) in 2017–2018 and 2018–2019, respectively (Table S13).
Discussion
In this cohort study of insured US adults, we estimated the annual economic burden of RSV infection on cardiorespiratory hospitalizations using an excess risk model and described differences in burden estimates when relying on ICD-10 diagnosis codes alone. There are at least two main takeaways from this study. First, modeling excess risk using viral positivity data provided a more complete estimation of RSV cardiorespiratory hospitalization burden and associated costs, compared with relying on ICD-10 diagnosis codes alone. Second, RSV contributes to substantial clinical and economic burden of cardiorespiratory hospitalizations among studied US adults and, to a greater extent, those at high risk of severe RSV. The results of this study can provide vaccine policy decision-makers and payers with a more complete understanding of the economic burden of RSV infections on cardiorespiratory hospitalizations among adults. This is particularly important to consider for those at high risk for severe RSV in an analysis of national-level data. Prior research has found that two of every three US adults aged ≥60 y have at least one underlying condition associated with an increased risk for severe RSV and that the prevalence of these conditions increases with age (1.7% of adults aged 20–49 y to 39.4% of adults ≥80 y having at least three underlying conditions).43 These data lend support to expanding RSV vaccination recommendations to adults at high risk for severe RSV, which could reduce the number of RSV cases and alleviate the economic burden of RSV infections on cardiorespiratory hospitalizations. This is consistent with findings from other modeling studies that predicted that effective RSV vaccination in adults aged ≥60 y could be beneficial to public health and reduce the economic burden of RSV infections.44–46
It is important to interpret the results of our study in the context of published literature. Most published studies have been limited to reporting direct costs of RSV infections, defined using laboratory confirmation or ICD diagnosis codes.10–14,47–51 Few studies have expanded beyond directly identifying confirmed and coded RSV infections by using ag broader case definition, such as cardiorespiratory or acute respiratory infection (ARI) diagnoses.19,52 Such methods can address undercoding for RSV, as well as more completely capturing medical events associated with RSV infection, including sequelae. Our findings are consistent with prior estimates of RSV burden among older adults, based on similar modeling methods used by Bosco and colleagues in the LTC setting that demonstrated a significant cost burden of RSV on cardiorespiratory hospitalizations.19 In contrast, Rafferty and associates reported RSV-attributable all-cause health care costs across all ages, but the authors used a retrospective case–control approach and varying RSV case definitions that resulted in a wide range of estimated costs.51 Our study expands on existing literature by extending to a broader US population and estimating a substantial RSV cost burden among adults across a wide age range. Furthermore, given that patients with high-risk conditions such as COPD, CAD, and HF have an RSV hospitalization incidence of up to 13, 7, and 33 times greater, respectively, compared with healthy adults, our study demonstrated that a large proportion of the clinical and economic burden of RSV infection on cardiorespiratory hospitalizations is represented among high-risk individuals.24
Methodologic differences make it difficult to compare estimates of RSV-associated health care costs across studies. Using similar methods to our study, Bosco and colleagues reported directionally consistent rates of ICD-coded RSV hospitalizations (5.81 per 100,000 PYs) and RSV-attributable cardiorespiratory hospitalizations (122, 95% CI: 93–151 per 100,000 PYs) across 2011–2017 among older adults in LTC facilities, with the RSV-attributable cardiorespiratory hospitalizations costing $51,503,105 (95% CI: $38,899,971–$64,106,240).19 Matias and associates also used similar methods to report consistent estimates of the average seasonal burden of RSV-attributable cardiorespiratory hospitalizations in years 1997–2009 (226,017 total, or 78 per 100,000 persons).18 Alternatively, Rafferty and colleagues used two case definitions for identifying RSV cases and controls across all age groups: (1) laboratory-confirmed RSV and (2) RSV-attributable ARIs during the RSV season.52 Health care costs attributable to RSV were estimated in Canadian dollars, as the mean difference in all-cause health care costs between RSV cases and controls. The authors reported substantial annual RSV-attributable costs per case across all ages ($40,028) using the laboratory-confirmed case definition, with the highest costs estimated for the youngest (age <2) and oldest (age ≥50) age groups. However, RSV-attributable costs per case across all ages were lower ($898) using the RSV-attributable ARI definition, compared with the cost per case for laboratory-confirmed RSV. The authors reasoned that laboratory testing for RSV is generally focused on severe cases and hospitalizations, and therefore, estimating RSV-associated health care costs based on laboratory-confirmed RSV or RSV-specific ICD diagnosis codes may overestimate costs for the majority of RSV cases. Future research using similar methods in other data sources and study populations, such as high-risk patients, should be conducted to contextualize findings from our study.
Strengths and limitations
This study has important strengths. First, MarketScan is a fit-for-purpose data source for this research question because it allowed both for robust economic analysis due to detailed health care utilization and cost data and for subgroup analysis due to the volume of data available. In addition, this study provided estimates of the economic burden of cardiorespiratory hospitalizations associated with RSV among a large segment of the adult population with health insurance by not relying only on ICD-coded RSV cases, which addresses an important gap in the literature.
This study also has important limitations. First, despite significant regional differences in the seasonality of RSV circulation,53 US geographic region is only available in the MarketScan database for individuals enrolled in commercial or Medicare supplemental health benefits. Also, to the best of our knowledge, the RSV laboratory positivity dataset from the CDC is not available at the state or region level. Thus, the study outcomes are unable to be stratified by US geographic region for Medicaid beneficiaries. Second, MarketScan lacks rural/urban status and socioeconomic status data, and race/ethnicity data are only available for a portion of individuals enrolled in Medicaid health benefits, precluding the assessment of particularly vulnerable populations. Third, this study includes individuals who were insured with continuous coverage of commercial, Medicare supplemental, or Medicaid health benefits. Individuals who are uninsured, insured under Medicare fee-for-service without supplemental coverage, or enrolled in a Medicare Advantage plan are not captured in this study. Thus, the Medicare population in this study is not representative of the larger proportion of Medicare-eligible US adults aged 65 y and older. Fourth, CDC surveillance data for RSV are collected on a weekly basis from participating public health and clinical laboratories across the US that voluntarily report specimen data; thus, the CDC data are not nationally representative, nor are the data representative within specific subgroups of individuals. Fifth, the inherent limitation of administrative claims data, primarily used for billing purposes, is a lack of detailed clinical information, such as the drug indication for immunosuppressive therapies. Given that our study aimed to estimate a more complete burden of RSV, our high-risk subgroup broadly included individuals with specific high-risk conditions or immunocompromised status, including those who recently received immunosuppressive therapies. Our approach used a claims-based algorithm developed by Polinski and colleagues27 that aligned with CDC guidelines for identifying moderately to severely immunocompromised individuals.54 Finally, it is important to acknowledge that there is no gold standard approach for estimating RSV-associated events, although several prior studies have used similar methods.18,19,55
Conclusions
In conclusion, we found that RSV infection contributes to the substantial clinical and economic burden of cardiorespiratory hospitalizations among US adults. Future research using other data sources and study populations (ie, Medicare fee-for-service) is needed to contextualize these results.
Supplementary Material
Acknowledgments
Editorial assistance was provided by MEDiSTRAVA in accordance with Good Publication Practice (GPP2022) guidelines, funded by Moderna, Inc., and under the direction of the authors.
Funding Statement
This research was funded by Moderna, Inc. This research received no external funding.
Disclosure statement
D.A.P., Z.A.M., A.C., A.T., and K.N. are employees at Aetion, Inc. D.A.P. and Z.A.M. are stock option holders of Aetion, Inc. C.A.P., P.G., S.S.L., and D.M. are employees of Moderna, Inc., and hold stock/stock options in the company.
Author contributions
Conceptualization, P.G, C.A.P., Z.A.M, D.A.P.; Methodology, Z.A.M., D.A.P., and A.C.; Software, Z.A.M, A.C., D.A.P., A.T., K.N.; Formal analysis, A.C., Z.A.M., D.A.P. A.T., K.N.; Writing – original draft preparation, D.A.P. and Z.A.M.; Writing – review and editing, D.A.P., Z.A.M., P.G., D.M., C.A.P., S.S.L.; Visualization, D.A.P and Z.A.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The WCG Institutional Review Board deemed this study exempt.
Data availability statement
MarketScan data have restrictions that apply due to the data license agreement. The weekly aggregated RSV laboratory positivity dataset used for excess risk modeling is openly available through request from the CDC NREVSS.
Informed consent statement
Patient consent was waived because this study utilized de-identified administrative claims data and weekly aggregated national laboratory surveillance data that provided no individual-level information.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2364493.
References
- 1.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE.. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–12. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 2.Korsten K, Adriaenssens N, Coenen S, Butler C, Ravanfar B, Rutter H, Allen J, Falsey A, Pirçon J-Y, Gruselle O, et al. Burden of respiratory syncytial virus infection in community-dwelling older adults in Europe (RESCEU): an international prospective cohort study. Eur Respir J. 2021;57(4):2002688. doi: 10.1183/13993003.02688-2020. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention . Learn about RSV in older adults with chronic medical conditions. 2024. Apr 12. [accessed 2024 Apr 12]. https://www.cdc.gov/rsv/high-risk/older-adults.html.
- 4.McLaughlin JM, Khan F, Begier E, Swerdlow DL, Jodar L, Falsey AR.. Rates of medically attended RSV among US adults: a systematic review and meta-analysis. Open Forum Infect Dis. 2022;9:ofac300. doi: 10.1093/ofid/ofac300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson NW, Binnicker MJ, Harris DM, Chirila RM, Brumble L, Mandrekar J, Hata DJ. Morbidity and mortality among patients with respiratory syncytial virus infection: a 2-year retrospective review. Diagn Microbiol Infect Dis. 2016;85(3):367–71. doi: 10.1016/j.diagmicrobio.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Chuaychoo B, Ngamwongwan S, Kaewnaphan B, Athipanyasilp N, Horthongkham N, Kantakamalakul W, Muangman N. Clinical manifestations and outcomes of respiratory syncytial virus infection in adult hospitalized patients. J Clin Virol. 2019;117:103–8. doi: 10.1016/j.jcv.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivey KS, Edwards KM, Talbot HK. Respiratory syncytial virus and associations with cardiovascular disease in adults. J Am Coll Cardiol. 2018;71:1574–83. doi: 10.1016/j.jacc.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Volling C, Hassan K, Mazzulli T, Green K, Al-Den A, Hunter P, Mangat R, Ng J, McGeer A. Respiratory syncytial virus infection-associated hospitalization in adults: a retrospective cohort study. BMC Infect Dis. 2014;14:665. doi: 10.1186/s12879-014-0665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi Y, Hill-Ricciuti A, Branche AR, Sieling WD, Saiman L, Walsh EE, Phillips M, Falsey AR, Finelli L. Cost determinants among adults hospitalized with respiratory syncytial virus in the United States, 2017–2019. Influenza Other Respir Viruses. 2022;16(1):151–8. doi: 10.1111/irv.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amand C, Tong S, Kieffer A, Kyaw MH. Healthcare resource use and economic burden attributable to respiratory syncytial virus in the United States: a claims database analysis. BMC Health Serv Res. 2018;18(1):294. doi: 10.1186/s12913-018-3066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belk K, Clark L, Mallow P, Banuelos R, Martin C. POSC190 hospital utilization for elderly patients diagnosed with respiratory syncytial virus (RSV) versus influenza in the US. Value in Health. 2022;25(1):S138. doi: 10.1016/j.jval.2021.11.664. [DOI] [Google Scholar]
- 12.Mesa-Frias M, Rossi C, Emond B, Bookhart B, Anderson D, Drummond S, Wang J, Lefebvre P, Lamerato LE, Lafeuille M-H, et al. Incidence and economic burden of respiratory syncytial virus among adults in the United States: a retrospective analysis using 2 insurance claims databases. J Manag Care Spec Pharm. 2022;28:753–65. doi: 10.18553/jmcp.2022.21459. [DOI] [PubMed] [Google Scholar]
- 13.Pastula ST, Hackett J, Coalson J, Jiang X, Villafana T, Ambrose C, Fryzek J. Hospitalizations for respiratory syncytial virus among adults in the United States, 1997–2012. Open Forum Infect Dis. 2017;4(1):ofw270. doi: 10.1093/ofid/ofw270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wyffels V, Kariburyo F, Gavart S, Fleischhackl R, Yuce H. A real-world analysis of patient characteristics and predictors of hospitalization among US medicare beneficiaries with respiratory syncytial virus infection. Adv Ther. 2020;37(3):1203–17. doi: 10.1007/s12325-020-01230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onwuchekwa C, Moreo LM, Menon S, Machado B, Curcio D, Kalina W, Atwell JE, Gessner BD, Siapka M, Agarwal N, et al. Underascertainment of respiratory syncytial virus infection in adults due to diagnostic testing limitations: a systematic literature review and meta-analysis. J Infect Dis. 2023;228(2):173–84. doi: 10.1093/infdis/jiad012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rozenbaum MH, Begier E, Kurosky SK, Whelan J, Bem D, Pouwels KB, Postma M, Bont L. Incidence of respiratory syncytial virus infection in older adults: limitations of current data. Infect Dis Ther. 2023;12(6):1487–504. doi: 10.1007/s40121-023-00802-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Z, Warren JL, Shapiro ED, Pitzer VE, Weinberger DM. Estimated incidence of respiratory hospitalizations attributable to RSV infections across age and socioeconomic groups. Pneumonia (Nathan). 2022;14(1):6. doi: 10.1186/s41479-022-00098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matias G, Taylor R, Haguinet F, Schuck-Paim C, Lustig R, Shinde V. Estimates of hospitalization attributable to influenza and RSV in the US during 1997–2009, by age and risk status. BMC Public Health. 2017;17(1):271. doi: 10.1186/s12889-017-4177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosco E, van Aalst R, McConeghy KW, Silva J, Moyo P, Eliot MN, Chit A, Gravenstein S, Zullo AR. Estimated cardiorespiratory hospitalizations attributable to influenza and respiratory syncytial virus among long-term care facility residents. JAMA Netw Open. 2021;4(6):e2111806. doi: 10.1001/jamanetworkopen.2021.11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melgar M, Britton A, Roper LE, Talbot HK, Long SS, Kotton CN, Havers FP. Use of respiratory syncytial virus vaccines in older adults: recommendations of the advisory committee on immunization practices — United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793–801. doi: 10.15585/mmwr.mm7229a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson E, Goswami J, Baqui AH, Doreski PA, Perez-Marc G, Zaman K, Monroy J, Duncan CJA, Ujiie M, Rämet M, et al. Efficacy and safety of an mRNA-based RSV PreF vaccine in older adults. N Engl J Med. 2023;389(24):2233–44. doi: 10.1056/NEJMoa2307079. [DOI] [PubMed] [Google Scholar]
- 22.Merative . Merative MarketScan Research Databases. 2022. [accessed 2024 Apr 12]. https://www.merative.com/content/dam/merative/documents/brief/marketscan-explainer-general.pdf.
- 23.National Center for Immunization and Respiratory Diseases (NCIRD) Division of Viral Diseases . RSV national trends - NREVSS. 2022. [accessed 2024 Apr 12]. https://www.cdc.gov/surveillance/nrevss/rsv/natl-trend.html.
- 24.Branche AR, Saiman L, Walsh EE, Falsey AR, Sieling WD, Greendyke W, Peterson DR, Vargas CY, Phillips M, Finelli L, et al. Incidence of respiratory syncytial virus infection among hospitalized adults, 2017-2020. Clin Infect Dis. 2022;74(6):1004–11. doi: 10.1093/cid/ciab595. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention . People at high risk of flu. 2023. Aug 25. [accessed 2024 Apr 12]. https://www.cdc.gov/flu/highrisk/index.htm.
- 26.National Center for Immunization and Respiratory Diseases . ACIP June 22-23, 2022 presentation slides - immunization practices. 2022. Jun. 23 [accessed 2024 Apr 12]. https://www.cdc.gov/vaccines/acip/meetings/slides-2022-06-22-23.html.
- 27.Polinski JM, Weckstein AR, Batech M, Kabelac C, Kamath T, Harvey R, Jain S, Rassen JA, Khan N, Schneeweiss S, et al. Durability of the single-dose Ad26.COV2.S vaccine in the prevention of COVID-19 infections and hospitalizations in the US before and during the delta variant surge. JAMA Netw Open. 2022;5(3):e222959. doi: 10.1001/jamanetworkopen.2022.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64:749–59. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gautam N, Bessette L, Pawar A, Levin R, Kim DH. Updating International Classification of Diseases 9th revision to 10th revision of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2021;76:1316–17. doi: 10.1093/gerona/glaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980–7. doi: 10.1093/gerona/glx229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun JW, Rogers JR, Her Q, Welch EC, Panozzo CA, Toh S, Gagne JJ. Adaptation and validation of the combined comorbidity score for ICD-10-CM. Med Care. 2017;55(12):1046–51. doi: 10.1097/MLR.0000000000000824. [DOI] [PubMed] [Google Scholar]
- 32.Keeney T, Flom M, Ding J, Sy M, Leung K, Kim DH, Orav J, Vogeli C, Ritchie CS. Using a claims-based frailty index to investigate frailty, survival, and healthcare expenditures among older adults hospitalized for COVID-19 at an academic medical center. J Frailty Aging. 2023;12:150–4. doi: 10.14283/jfa.2023.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matias G, Taylor R, Haguinet F, Schuck-Paim C, Lustig R, Shinde V. Estimates of mortality attributable to influenza and RSV in the United States during 1997–2009 by influenza type or subtype, age, cause of death, and risk status. Influenza Other Respir Viruses. 2014;8(5):507–15. doi: 10.1111/irv.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McConeghy KW. Modified Serfling models. 2019. Jun 6. [accessed 2024 Apr 12]. https://kmcconeghy.github.io/flumodelr/articles/05-modserf.html.
- 35.Centers for Disease Control and Prevention . RSV-NET overview and methods. 2022. Oct 25. [accessed 2024 Apr 12]. https://www.cdc.gov/rsv/research/rsv-net/overview-methods.html#:~:text=Case%20Definition,-A%20case%20is&text=Evidence%20of%20RSV%20infection%20can,paired%20acute%20and%20convalescent%20specimens.
- 36.Aetion . Software for real-world data analysis. 2021. [accessed 2024 Apr 12]. https://aetion.com/.
- 37.R Core Team . A language and environment for statistical computing. 2021. [accessed 2024 Apr 12]. https://www.R-project.org/.
- 38.Froes F, Carmo M, Lopes H, Bizouard G, Gomes C, Martins M, Bricout H, de Courville C, de Sousa JC, Rabaçal C, et al. Excess hospitalizations and mortality associated with seasonal influenza in Portugal, 2008–2018. BMC Infect Dis. 2022;22(1):726. doi: 10.1186/s12879-022-07713-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.US Bureau of Labor Statistics . How BLS measures price change for medical care services in the consumer price index 2023. [accessed 2024 Apr 12]. https://www.bls.gov/cpi/factsheets/medical-care.htm.
- 40.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–25. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 41.Howe CJ, Cole SR, Lau B, Napravnik S, Eron JJ Jr. Selection bias due to loss to follow up in cohort studies. Epidemiology. 2016;27(1):91–7. doi: 10.1097/EDE.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim DH, Glynn RJ, Avorn J, Lipsitz LA, Rockwood K, Pawar A, Schneeweiss S. Validation of a Claims-Based Frailty Index Against Physical Performance and Adverse Health Outcomes in the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2019. Jul 12;74(8):1271–1276. doi: 10.1093/gerona/gly197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghaswalla P, Hitchens A, Candrilli SD, Carrico J, Hicks KA, Wilson E, Mehta D, Panozzo CA. 903. Prevalence of underlying conditions associated with higher risk for severe RSV, influenza, or COVID-19 in adults in the United States, 2017-2018. Open Forum Infect Dis. 2023;10(Supplement_2):ofad500.948. doi: 10.1093/ofid/ofad500.948. [DOI] [Google Scholar]
- 44.Postma MJ, Cheng CY, Buyukkaramikli NC, Hernandez Pastor L, Vandersmissen I, Van Effelterre T, Openshaw P, Simoens S. Predicted public health and economic impact of respiratory syncytial virus vaccination with variable duration of protection for adults ≥60 years in Belgium. Vaccines (Basel). 2023;11(5):990. doi: 10.3390/vaccines11050990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fust K, Kohli M, Panozzo CA, Weinstein MC, Buck P, Ghaswalla P. 116. Potential clinical impact of respiratory syncytial virus (RSV) vaccination in older adults in the United States. Open Forum Infect Dis. 2022;9(Supplement_2). doi: 10.1093/ofid/ofac492.194. [DOI] [Google Scholar]
- 46.Van Effelterre T, Hens N, White LJ, Gravenstein S, Bastian AR, Buyukkaramikli N, Cheng CY, Hartnett J, Krishnarajah G, Weber K, et al. Modeling respiratory syncytial virus adult vaccination in the United States with a dynamic transmission model. Clin Infect Dis. 2023;77(3):480–9. doi: 10.1093/cid/ciad161. [DOI] [PubMed] [Google Scholar]
- 47.Ackerson B, An J, Sy LS, Solano Z, Slezak J, Tseng HF. Cost of hospitalization associated with respiratory syncytial virus infection versus influenza infection in hospitalized older adults. J Infect Dis. 2020;222:962–6. doi: 10.1093/infdis/jiaa183. [DOI] [PubMed] [Google Scholar]
- 48.Chen L, Han X, Li Y, Zhang C, Xing X. Comparison of clinical characteristics and outcomes between respiratory syncytial virus and influenza-related pneumonia in China from 2013 to 2019. Eur J Clin Microbiol Infect Dis. 2021;40(8):1633–43. doi: 10.1007/s10096-021-04217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Sherif M, McNeil S, Andrew M. Prevalence, severe outcomes, and costs associated with respiratory syncytial virus (RSV) in adults 50 years of age hospitalized with respiratory illness in Canada, 2012-2015. Abstract no. E&E-17 6th ReSViNET Conference; November 10-12; Online; 2021. Virtual Conference. [Google Scholar]
- 50.Prasad N, Newbern EC, Trenholme AA, Thompson MG, McArthur C, Wong CA, Jelley L, Aminisani N, Huang QS, Grant CC, et al. The health and economic burden of respiratory syncytial virus associated hospitalizations in adults. PLoS One. 2020;15(6):e0234235. doi: 10.1371/journal.pone.0234235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoon JG, Noh JY, Choi WS, Park JJ, Suh YB, Song JY, Cheong HJ, Kim WJ. Clinical characteristics and disease burden of respiratory syncytial virus infection among hospitalized adults. Sci Rep. 2020;10(1):12106. doi: 10.1038/s41598-020-69017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rafferty E, Paulden M, Buchan SA, Robinson JL, Bettinger JA, Kumar M, Svenson LW, MacDonald SE. Evaluating the individual healthcare costs and burden of disease associated with RSV across age groups. Pharmacoeconomics. 2022;40(6):633–45. doi: 10.1007/s40273-022-01142-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGuiness CB, Boron ML, Saunders B, Edelman L, Kumar VR, Rabon-Stith KM. Respiratory syncytial virus surveillance in the United States, 2007-2012: results from a national surveillance system. Pediatr Infect Dis J. 2014;33(6):589–94. doi: 10.1097/inf.0000000000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention . Interim clinical considerations for use of COVID-19 vaccines in the United States. 2024. Apr 4. [accessed 2024 May 3]. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html.
- 55.Thompson WW, Weintraub E, Dhankhar P, Cheng PY, Brammer L, Meltzer MI, Bresee JS, Shay DK. Estimates of US influenza-associated deaths made using four different methods. Influenza Other Respir Viruses. 2009;3(1):37–49. doi: 10.1111/j.1750-2659.2009.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
MarketScan data have restrictions that apply due to the data license agreement. The weekly aggregated RSV laboratory positivity dataset used for excess risk modeling is openly available through request from the CDC NREVSS.


