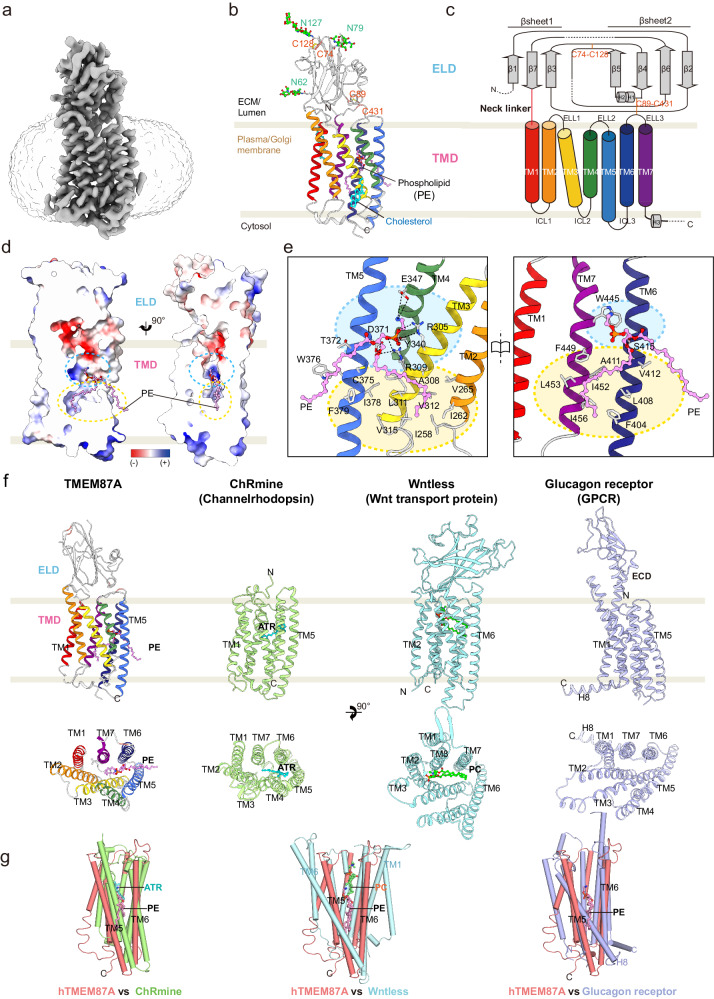
Fig. 3. Cryo-EM structure and structural feature of human TMEM87A.
a Cryo-EM density map of hTMEM87A, colored in slate gray. The density of the micelle (contoured at 0.161σ) is presented as light gray. b Overall structure of hTMEM87A, with ELD (light gray) and TMD [rainbow color from TM1 (red) to TM7 (purple)]. Disulfide bridges (orange, C74–C128, and C89–C431) and N-linked glycans (green, N62, N79, and N127) are shown as sticks. PE and cholesterol are indicated as pink and cyan sticks, respectively. c Topology of hTMEM87A. ELD consists of two α-helices and seven β-strands arranged in an anti-parallel β-sandwich. The secondary structure elements (cylinder for helix and arrow for strand) are colored as in (b). Disulfide bonds are shown as an orange line. Dashed lines denote regions where density was insufficient for model building. d Two different views of the vertical cross-section of the PE-binding pocket in TMD. The electrostatic surface potential of the central cavity is shown. The upper hydrophilic and lower hydrophobic cavities are indicated as cyan and yellow dashed circles, respectively. e Open-book views of the PE-binding pocket and the interaction details. Interaction residues with PE are shown as sticks. The hydrogen and ionic bonds are depicted as a dashed line. Cyan and yellow colored circles represent hydrophilic and hydrophobic cavities in TMD, respectively. f Structural comparison of hTMEM87A TMD with other seven transmembranes (7TM) proteins ChRmine (PDB:7W9W, pale green), Wntless (PDB:7DRT, cyan), and Glucagon receptor (PDB:5YQZ, light purple), shown as a side view (top) and top view (bottom). In the top view, ELD (hTMEM87A), luminal domain (LD, Wntless), and extracellular domain (ECD, glucagon receptor) are omitted for clarity. PE in hTMEM87A, ATR in ChRmine, and Phosphatidylcholine (PC) in Wntless are displayed as pink, cyan, and lime sticks, respectively. g Superimposition of hTMEM87A TMD and TM region of ChRmine (pale green), Wntless (cyan), or glucagon receptor (light purple). The view is a 90° rotation view of (b). along the y-axis to show the lateral opening between TM5 and TM6 of hTMEM87A TMD.