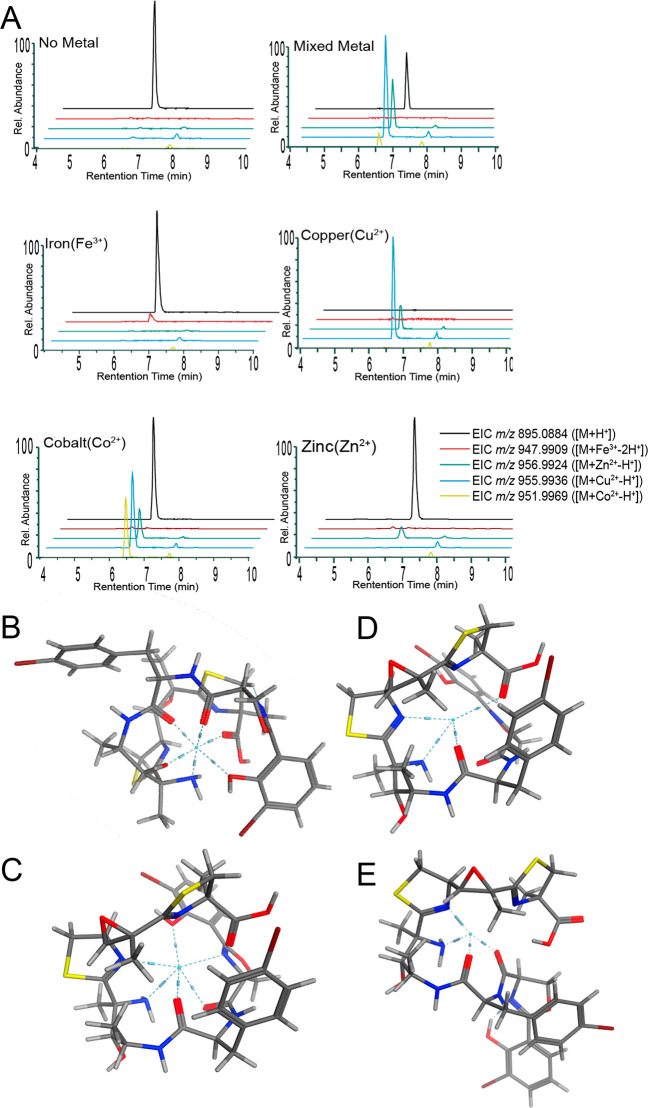
Figure 5.
Metal binding properties of leptochelin A (1). A. Extracted ion chromatograms from metal-binding studies using pure leptochelin A (1). Note that the cobalt injection shows small peaks for copper and zinc adducts. These latter peaks may result from in-instrument contamination. Molecular modeling in MOE (Amber10:EHT) as seen in B - E indicates that the carbonyl groups, amine group, phenolic oxygen, and nitrogen atoms of the thiazoline and oxazoline rings participate in the hexadentate and tetradentate coordination of metals. B. Modeled pose of iron(III) complexed with leptochelin A (1); coordinating residues: C-7 N, C-10 N, C-17 O, and C-26 O. C. Modeled pose of cobalt(II) complexed with leptochelin A (1); coordinating residues: C-7 N, C-10 N, C-17 O, C-26 O, C-27 N, and C-31 O. D. Modeled pose of copper(II) complexed with leptochelin A (1); coordinating residues: C-1 O, C-7 N, C-10 N, and C-17 O. E. Modeled pose of zinc(II) complexed with leptochelin A (1); coordinating residues: C-7 N, C-10 N, C-17 O, and C-26 O. Additionally, when coordinated with metals, the leptochelins produce a pseudocyclic conformation, an observation that is consistent with through-space correlations observed by ROESY NMR with zinc-bound leptochelin A (1) (e.g., ROE from H3-13 to H-27, Table 1, SI Appendix, Figure S51).