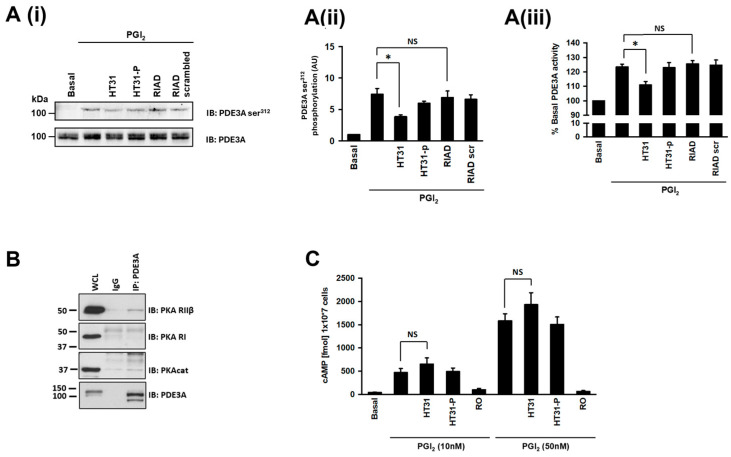
Figure 3.
Effect of AKAP disruption on PDE3A activity and phosphorylation. (A) Washed human platelets (5 × 108 platelets/mL) were incubated with either non-specific AKAP disruptor peptide HT31 (2 μM), the HT31 control peptide HT31-P (2 μM), PKA RI specific disruptor peptide, RIAD (2.5 μM), and RIAD control peptide RIAD-scrambled (2.5 μM) for 60 min prior to stimulation with PGI2 (100 nM, 2 min). Samples were immunoblotted for PDE3A ser312 phosphorylation and PDE3A as a loading control and analysed for PDE3A activity. (A(i)) Representative blots of 3 independent experiments. (A(ii)) PDE3A ser312 phosphorylation densitometric analysis of 3 independent experiments expressed as arbitrary units (AU) * p < 0.05. NS, not significant. (A(iii)) As in (A(ii)), except PDE3A immunoprecipitates were assayed for PDE3A activity (* p < 0.05). (B) Human platelet lysates (500 mg proteins) were incubated with PDE3A(CT) antibody (2 μg) to immunoprecipitate PDE3A. The PDE3A immunoprecipitates were then blotted for PKA RI and PKA RII on separate membranes. Immunoblots were stripped and reprobed for PDE3A as a loading control and PKA catalytic subunit (PKAcat) as a positive control. Representative blots from 3 independent experiments. (C) As in A, except human platelets were either pre-treated with 10 nM or 50 nM PGI2, lysed, and cAMP measured. In some cases, human platelets were preincubated with the IP receptor antagonist (RO1138452; 100 µM) prior to PGI2. Data are expressed as cAMP (fmol/107 platelets) (n = 4).