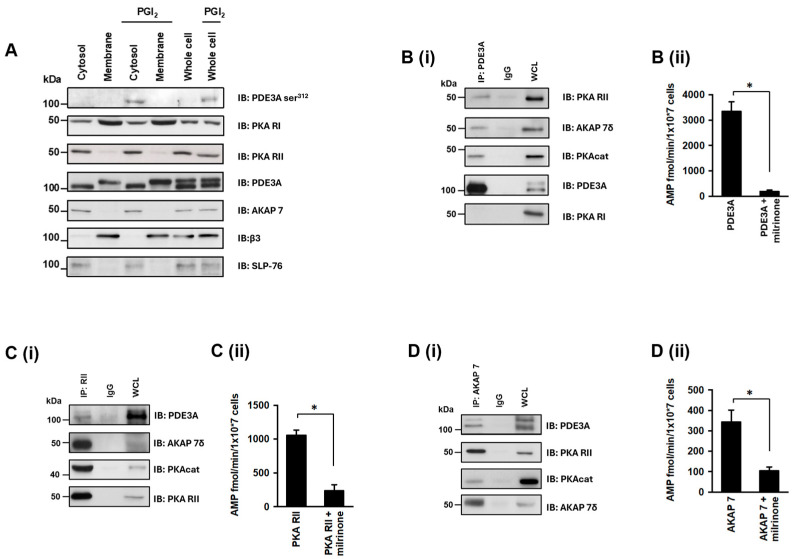
Figure 5.
Identification of a PKA RII/AKAP7/PDE3A signalling complex in human platelets. (A) Washed human platelets (7 × 108 platelets/mL) were stimulated with PGI2 (100 nM, 2 min), lysed, and centrifuged into membrane and cytosolic fractions. Fractions were immunoblotted for phosphoPDE3A ser312, PKA RI, PKA RII, AKAP7, β3, and SLP-76. (B) Washed human platelet lysates (1 mg proteins) were incubated with PDE3A antibody (5 μg) covalently coupled to an amine-reactive resin. PDE3A immunoprecipitates were immunoblotted for PKA RII and PKA RI and then reprobed for PDE3A as a loading control. Similarly, human platelet lysates (1 mg proteins) were incubated with (C) PKA RII antibody (5 μg), and (D) AKAP7 antibody (5 μg) covalently coupled to amine-reactive resin. Following incubation, co-immunoprecipitated proteins were isolated and immunoblotted for (C(i)) PDE3A, AKAP7, PKAcat, and PKA RII; and (D(i)) PDE3A, PKA RII, PKAcat, and AKAP7. Representative immunoblots of 3 independent experiments. (B(ii),C(ii),D(ii)) Respective co-immunoprecipitated proteins (500 μg protein) were assayed for PDE3A activity, in the presence and absence of milrinone (10 μM, 20 min). Data representative of 3 independent experiments * p < 0.01.