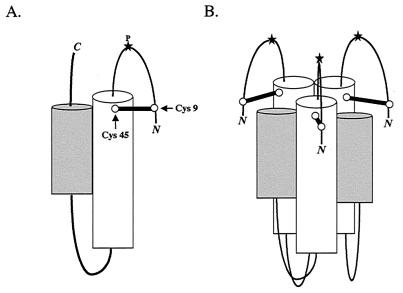
FIG. 6.
Model of a fusion-active state of the ASLV TM trimer. The core monomer and six-helix bundle formed by the gp37 trimer were modeled after the crystal structures of the core fragment of Ebola virus GP2 (13a, 15). (A) The gp37 monomer. The predicted N-terminal (white) and C-terminal (gray) α-helices pack against each other in an antiparallel orientation, placing the N-terminal loop (containing the fusion peptide) and the C-terminal portions of the protein near each other, extending toward the common fused membrane. A star at the apex of the loop (P) denotes the predicted position of the proline within the fusion peptide. The loop containing the proline is shown stabilized by a disulfide bond between C9 (Cys9) and C45 (Cys45). The disulfide-bonded cysteines are represented by a dumbbell and marked by arrows. The N and C termini are marked. (B) The gp37 trimer. By analogy to other trimeric viral fusion proteins, the N-terminal helices are expected to pack against each other, forming a threefold symmetry axis down the center of the resulting coil (13a, 15) with a given C-terminal helix resting in the groove made by its own and a neighboring N-terminal helix. The C-terminal residues extending from these helices have been omitted for clarity.