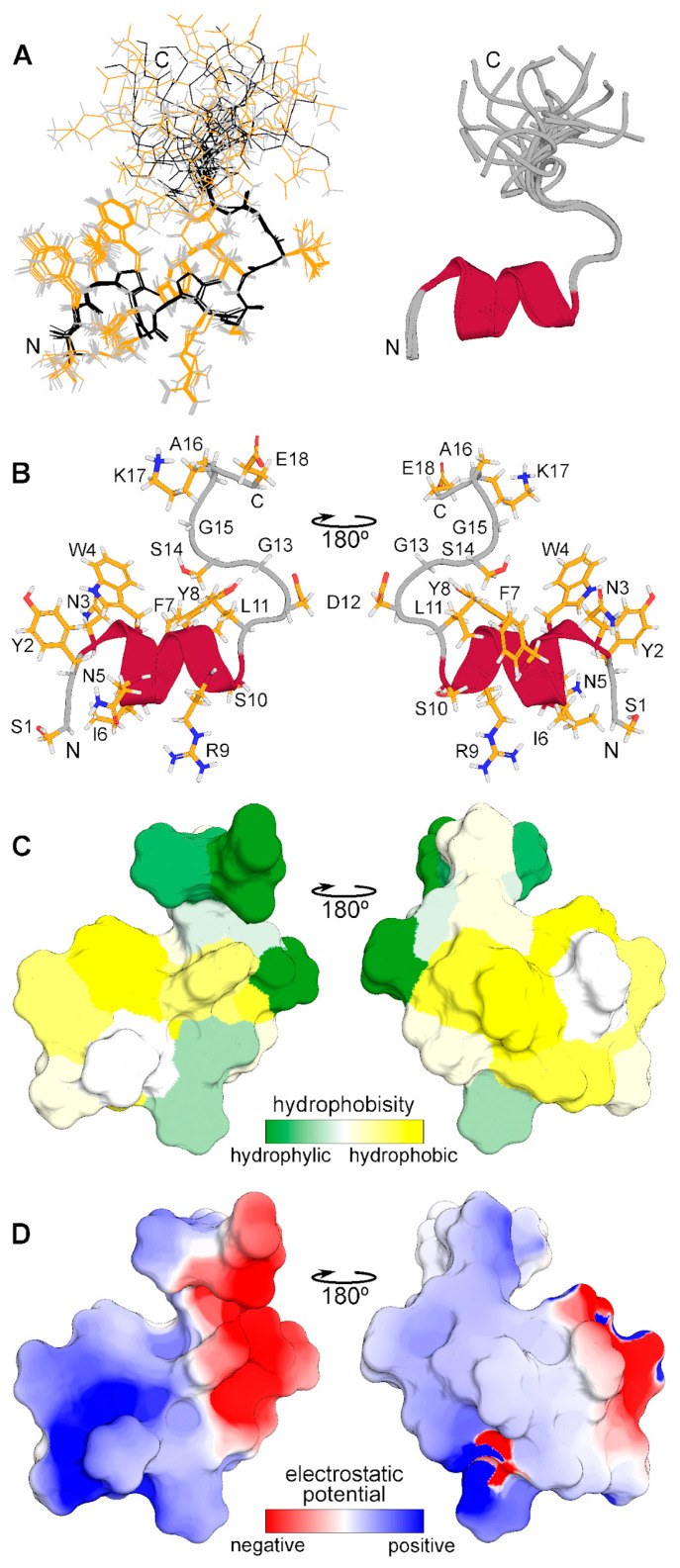
Figure 4.
Spatial structure of CpRE12 obtained by NMR analysis in DPC micellar environment. (A) Superposition of 12 NMR structures with the lowest target function aligned over the backbone atoms of the folded N-terminal helical part (residues 1–14). Backbone and side chain heavy atom bonds are shown in black and yellow, respectively. Superimposed ribbon diagrams of the NMR-derived structures of are presented on the right. (B) Representative NMR-derived structure of CpRE12. (C) Molecular hydrophobicity potential (MHP) distribution on the CpRE12 surface. Green is the most hydrophilic (MHP ≤ −3.6), yellow is the most hydrophobic (MHP ≥ 2.1). MHP values are given in logP units, where P is the octanol/water partition coefficient. (D) Molecular electrostatic potential (MEP) distribution on the CpRE12 surface. Red is the most negative (MEP ≤ −3 kt/e), blue is the most positive (MEP ≥ 3 kt/e) potential.