Abstract
Background
We observed rapid tumor progression following COVID-19 infection among patients with glioblastoma and sought to systematically characterize their disease course in a retrospective case–control study.
Methods
Using an institutional database, we retrospectively identified a series of COVID-19-positive glioblastoma cases and matched them by age and sex 1:2 to glioblastoma controls who had a negative COVID-19 test during their disease course. Demographic and clinical data were analyzed. Hyperprogression was defined using modified response evaluation criteria in solid tumors criteria. Time to progression and overall survival were estimated using the Kaplan–Meier method.
Results
Thirty-two glioblastoma cases with positive COVID-19 testing were matched to 64 glioblastoma controls with negative testing; age, sex, and molecular profiles did not differ between groups. Progression events occurred in 27 cases (84%) and 46 controls (72%). Of these, 14 cases (52%) presented with multifocal disease or leptomeningeal disease at progression compared with 10 controls (22%; P = .0082). Hyperprogression was identified in 13 cases (48%) but only 4 controls (9%; P = .0001). Cases had disease progression at a median of 35 days following COVID-19 testing, compared with 164 days for controls (P = .0001). Median survival from COVID-19 testing until death was 8.3 months for cases but 17 months for controls (P = .0016). Median overall survival from glioblastoma diagnosis was 20.7 months for cases and 24.6 months for controls (P = .672).
Conclusions
Patients with glioblastoma may have accelerated disease progression in the first 2 months after COVID-19 infection. Infected patients should be monitored vigilantly. Future investigations should explore tumor-immune microenvironment changes linking tumor progression and COVID-19.
Keywords: COVID-19, glioblastoma, glioma, progression, SARS-CoV-2
As of March 2023, 6.8 million worldwide deaths were attributable to the COVID-19 pandemic,1 which has impacted medical care and outcomes for all patients. Studies regarding COVID-19 in patients with glioma have generally focused on immunologic vulnerability to the virus2 and the potential for worse outcomes related to barriers in treatment delivery.3 For example, an investigation by Aaroe et al. revealed a high incidence of disruptions to glioma therapy plans, including delayed surgery and interruptions to radiation or chemotherapy, that were attributable to COVID-19 infection.4
Less is known about the potential direct effects of the SARS-CoV-2 virus on central nervous system (CNS) tumor biology. A high proportion of patients with COVID-19 develop neurologic symptoms (up to 45% of severely affected patients, according to an early case series),5 and mechanisms of SARS-CoV-2 neurotropism are being increasingly elucidated.6,7 Neurotoxicity from COVID-19 has been proposed to arise in part from S1 subunit-induced MAPK pathway activation, NF-κB signaling, and p53 activation, which can induce inflammatory and neurodegenerative changes.8
A variety of coronavirus receptors, including ACE2,9 ANPEP, and ENPEP, have been shown to have higher expression in glioblastoma tissues than in normal tissues; ANPEP and ENPEP were associated with increased immune-cell infiltration of monocytes and poor prognosis in glioblastoma.10 Several publications have arisen in recent months exploring the profound and enduring dysregulation of inflammation and the myeloid cell compartment specifically in COVID-19,11–13 which may reshape the tumor microenvironment and immunophenotype.
In this context, we recognized a trend of unexpectedly rapid tumor progression in our practice following COVID-19 infection among patients with glioblastoma. Thus, we sought to systematically characterize the pace of progression in an unselected cohort of all patients with glioblastoma and COVID-19 seen in our center since the beginning of the COVID-19 pandemic in a retrospective case–control study.
Materials and Methods
Patient Selection
Patients with glioblastoma who had a positive COVID-19 test result between April 2020 and March 2023 were retrospectively selected for analysis using The University of Texas MD Anderson Cancer Center D3CODE (Data-Driven Determinants for COVID-19 Oncology Discovery Effort) and PROACTIVE-glioma-program (Prospective Assessment of Correlative and Tissue Biomarkers in Glioma Patients) database initiatives under MD Anderson IRB-approved protocols (2020-0348 and 2012-0441) and a waiver of informed consent was obtained. During the study period, COVID-19 testing was routinely performed as a protocol at MD Anderson. COVID-19 testing was also performed for patients with respiratory symptoms. None of the COVID-19 tests were prompted by new neurological symptoms. The Epic electronic medical record search tool (SlicerDicer) was used to verify that the cohort was complete and to identify control patients with glioblastoma who had a negative COVID-19 test result between April 2020 and March 2023. Controls and cases were selected in a 2:1 ratio and matched by age and sex. Demographic, clinical, and survival data, including molecular and radiographic features, were obtained by chart review. Patients were included if histologic examination revealed glioblastoma with wild-type IDH status and excluded if molecular testing was consistent with an alternative WHO CNS entity. Molecular profiles, which were obtained using the MD Anderson Solid Tumor Genomic Assay, and MGMT promoter methylation status were annotated when available. An additional subset of patients was noted to develop the first clinical signs of new glioblastoma after COVID-19 infection, and for these patients, the time interval from positive COVID-19 test to glioblastoma diagnosis was recorded.
Data Analysis
Descriptive statistics (frequency distribution, median, and range) were used to summarize patient characteristics. Time to progression was defined as the time from the date of the COVID-19 test to the time of disease progression or death, whichever occurred first. Progression was defined as the first imaging demonstration of at least a 25% increase in the product of the perpendicular diameters using Response Assessment in Neuro-Oncology (RANO) criteria14 or new nodular disease. Hyperprogression was defined using modified Response Evaluation Criteria in Solid Tumors (RECIST), requiring presentation of 3 or more of the following items: disease progression within 2 months of COVID-19 test, increase of at least 50% in the primary measurable lesion compared with prior baseline, progression with multifocality or leptomeningeal disease, or clinical deterioration at progression (as indicated by a drop in Karnofsky Performance Status or development of symptoms clearly referable to disease).15 Overall survival (OS) was defined as the time from the diagnosis of glioblastoma at initial surgery to the time of death. For events that had not occurred by the time of data analysis, times were censored at the time of last contact at which the patient was known to be progression-free (for time to progression) or to be alive (for OS). The distributions of the time-to-event outcomes were estimated using the Kaplan–Meier method, and log-rank testing was performed to assess the difference in time to progression and OS between groups.16,17 A P -value < .05 was considered statistically significant. To reduce lead-time bias, time to progression and survival analyses were stratified according to the length of time from glioblastoma diagnosis into groups that had a positive or negative COVID-19 test result during the first year (Y1), second year (Y2), or third year and beyond (Y3+) since diagnosis.
Results
Clinical Characteristics
In this study, 32 patients with glioblastoma and a positive COVID-19 test result during their disease course were identified and matched with 64 controls with glioblastoma and a negative COVID-19 test result. Cases and controls had no significant differences in terms of age, sex, or molecular glioblastoma profile (Table 1). Median age was 56 years (range 27–79 years) for cases and 55 years (range 20–78 years) for controls. Case and control groups were each 53% male. A complete molecular profile and MGMT status were available for 29 cases (91%) and 60 controls (94%). A number of gene alterations, prevalence of TERT promoter mutations, PI3K/MAPK pathway alterations, and MGMT methylation status were not significantly different between cases and controls. Regarding COVID-19 vaccination, 14 cases (44%) and 40 controls (63%) had no record of vaccination (P = .08). Among the infected cases, the severity of COVID-19 infection ranged from asymptomatic to moderate, with a minority requiring hospitalization and no cases requiring intubation.
Table 1.
Demographic and Clinical Characteristics of IDH-Wildtype Glioblastoma Cases and Controls
| COVID-19(+) Cases (n = 32) | COVID-19(–) Controls (n = 64) | P | |
|---|---|---|---|
| Median age (range), y | 56 (27–79) | 55 (20–78) | .48 |
| Sex, no. (%) | .83 | ||
| Male | 17 (53) | 34 (53) | |
| Female | 15 (47) | 30 (47) | |
| COVID-19 vaccination status prior to COVID test, no. (%) | .08 | ||
| 1–2 doses | 10 (31) | 18 (28) | |
| Boosted | 8 (25) | 6 (9) | |
| No record | 14 (44) | 40 (63) | |
| Molecular profiling | |||
| Sequencing and MGMT status-completed—no. (%) | 29 (91) | 60 (94) | .58 |
| Tumor gene alterations—median (range) | 4 (1–9) | 4 (1–15) | |
| TERT promoter mutation—no. (%) | 23 (77) | 42 (70) | .54 |
| PI3K/MAPK alteration—no. (%) | 27 (90) | 55 (90) | .84 |
| No. of unique PI3K/MAPK alterations—median (range) | 1 (0–4) | 1 (0–4) | |
| MGMT methylated—no. (%) | 14 (47) | 27 (44) | .88 |
Progression Characteristics
A documented progression event occurred in 27 cases (84%) and 46 controls (72%; P = .18; Table 2; Figure 1). These progression events varied; we observed 19 cases (70%) and 12 controls (26%) who had disease progression within 2 months of COVID-19 testing (P = .00022), 13 cases (48%) and 14 controls (30%) with at least a 50% increase in tumor area (P = .13), 14 cases (52%) and 10 controls (22%) with multifocality or leptomeningeal disease (P = .0082), and 17 cases (63%) and 19 controls (41%) who experienced clinical deterioration at progression (P = .074). Of those who experienced progression, 13 cases (48%) and 4 controls (9%) met the criteria for hyperprogression (P = .00037).
Table 2.
Characteristics of Progression and Survival
| COVID-19(+) Cases (n = 32) | COVID-19(–) Controls (n = 64) | P | |
|---|---|---|---|
| Progression event during follow-up, no. (%) | 27 (84) | 46 (72) | .18 |
| Hyperprogression criteria satisfied, no. (%) | 13 (48) | 4 (9) | <.001 |
| Progression within 2 months of COVID-19 testing | 19 (70) | 12 (26) | <.001 |
| >50% increase in tumor area | 13 (48) | 14 (30) | .13 |
| Presented with multifocality or LMD | 14 (52) | 10 (22) | .008 |
| Clinical deterioration at progression | 17 (63) | 19 (41) | .074 |
| Time from COVID test to progression for all, median days | 35 (1–734) | 164 (2–882) | <.001 |
| Y1 group | 35 | 117 | .075 |
| Y2 group | 35 | 301 | <.001 |
| Y3+ group | 47 | 119 | .23 |
| Time from COVID test to death for all, median months | 8.3 | 17 | .002 |
| Y1 group | 7.6 | 13.7 | .064 |
| Y2 group | 4.3 | 18.4 | <.001 |
| Y3+group | 12.4 | NR | |
| Overall survival for all who progressed, median months | 20.7 | 24.6 | .67 |
| Y1 group | 8.9 | 13 | .004 |
| Y2 group | 21.1 | 23.8 | .15 |
| Y3+group | 66.7 | NR |
Y1, Y2, and Y3+ patients underwent COVID-19 testing during the first year, second year, and third or more years after GBM diagnosis, respectively. Y1 cases n = 7, controls n = 20; Y2 cases n = 9, controls n = 22; Y3+cases n = 8, controls n = 9. Significant values are bolded.
Figure 1.
T1 + C MRI images from select glioblastoma cases at baseline before COVID-19 infection (top) and with notable progression 10–36 days after a positive COVID-19 test (bottom).
Survival Characteristics
Time to progression.
—Cases had disease progression at a median of 35 days (range 1–734 days) following a positive COVID-19 test result, whereas controls had disease progression at a median of 164 days (range 2–882 days) following a negative test result (P = .0001; Figure 2). When stratifying by the number of years from glioblastoma diagnosis to COVID-19 test, median time to progression was 35 days for Y1 cases and 117 days for Y1 controls (P = .0753), 35 days for Y2 cases and 301 days for Y2 controls (P = .0001), and 47 days for Y3+ cases and 119 days for Y3+ controls (P = .2286).
Figure 2.
Time (days) from COVID-19 test to progression for A, all cases and controls; B, cases and controls who underwent COVID-19 testing during the first year (Y1) after diagnosis of glioblastoma; C, cases and controls who underwent COVID-19 testing during the second year (Y2) after diagnosis of glioblastoma; and D, cases and controls who underwent COVID-19 testing during the third year or beyond (Y3+) after diagnosis of glioblastoma.
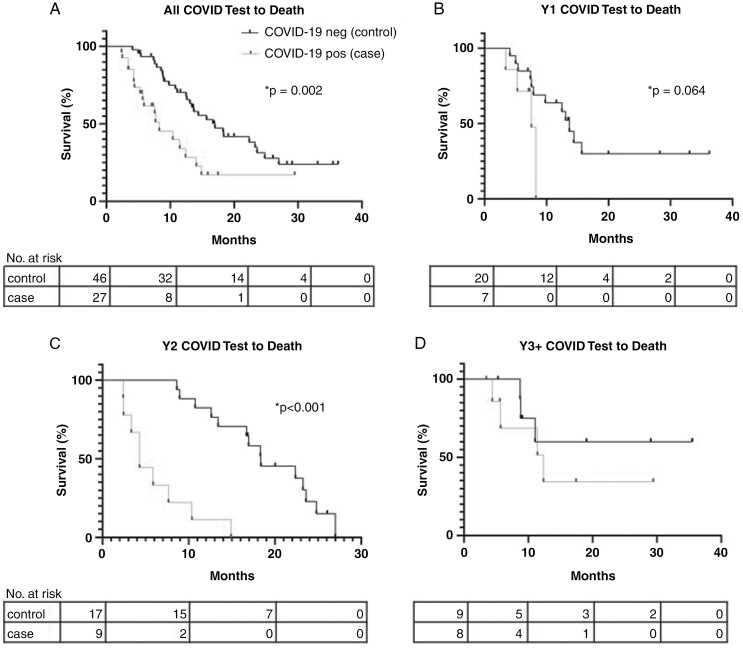
Time from COVID-19 test to death.
—Median survival from the time of COVID-19 test until death was 8.3 months for cases but 17 months for controls (P = .0016; Figure 3). When stratifying by the number of years from glioblastoma diagnosis to COVID-19 test, median survival was 7.6 months for Y1 cases and 13.7 months for Y1 controls (P = .064), 4.3 months for Y2 cases and 18.4 months for Y2 controls (P = .0001), and 12.4 months for Y3+ cases; median survival for Y3+ controls was not reached.
Figure 3.
Time (months) from COVID-19 test to death for A, all cases and controls; B, cases and controls who underwent COVID-19 testing during the first year (Y1) after diagnosis of glioblastoma; C, cases and controls who underwent COVID-19 testing during the second year (Y2) after diagnosis of glioblastoma; and D, cases and controls who underwent COVID-19 testing during the third year or beyond (Y3+) after diagnosis of glioblastoma.
Overall survival from glioblastoma diagnosis.
—Median OS from glioblastoma diagnosis to death was 20.7 months for cases and 24.6 months for controls (P = .672; Figure 4). When stratifying by the number of years from glioblastoma diagnosis to COVID-19 test, median OS was 8.9 months for Y1 cases and 13 months for Y1 controls (P = .0042), 21.1 months for Y2 cases and 23.8 months for Y2 controls (P = .1472), and 66.7 months for Y3+ cases; median OS for Y3+ controls was not reached.
Figure 4.
Overall survival (months) of patients who had disease progression for A, all cases and controls; B, cases and controls who underwent COVID-19 testing during the first year after (Y1) diagnosis of glioblastoma; C, cases and controls who underwent COVID-19 testing during the second year (Y2) after diagnosis of glioblastoma; and D, cases and controls who underwent COVID-19 testing during the third year or beyond (Y3+) after diagnosis of glioblastoma.
New glioblastoma diagnoses preceded by COVID-19 infection.
—We observed that 7 patients developed initial presenting symptoms of glioblastoma shortly after COVID-19 infection. The median time to presentation after a positive COVID-19 test result was 17 days (range 0–104 days), which occurred at a median age of 54 years (range 44–64 years). Of these 7 patients, 3 patients developed cognitive changes, 2 patients developed headaches, 1 patient developed seizures, and 1 patient developed focal weakness as their initial presenting symptoms. One of the 7 patients had a documented COVID-19 vaccine prior to diagnosis.
Discussion
In this retrospective case–control study of the effects of COVID-19 in patients with glioblastoma, we observed accelerated tumor progression, including more aggressive tumor behavior, faster time to progression, more rapid decline to death, and reduced OS, after COVID-19 infection. To our knowledge, this is the first analysis of glioblastoma progression following COVID-19 infection.
Enduring dysregulation of the systemic immune system following COVID-19 infection has been well documented. For example, evidence of sustained immune activation and markers of exhaustion of CD4+ and CD8+ T cells in convalescent blood samples were seen at a median of 29 days after COVID-19 infection,18 and high levels of inflammatory gene expression among CD14+ monocytes and decreased CD8+ T cells were found during COVID-19 recovery.19 A study using immunophenotyping, RNA sequencing, and serum cytokine analysis showed that increased oxidative phosphorylation-associated inflammatory signatures and disrupted TNF and IL-6 responses persisted for up to 60 days after patients first experienced COVID-19 symptoms.20 A prolonged anti-spike and anti-receptor-binding-domain humoral response has been observed for at least 6 months after COVID-19 infection, and this was associated with profound alterations in the immune-cell population that peaked 12–16 weeks after infection.12 Our observations of aggressive glioblastoma activity at a median of 35 days following a positive COVID-19 test may suggest that immune disruption contributed to glioblastoma permissiveness and dysregulated inflammation.
In addition to systemic immune changes, the direct effects of the SARS-CoV-2 virus on the CNS may contribute to a tumor environment that permits growth and inflammation. It has been hypothesized that systemic inflammation and astrocytic injury during infection are sufficient to disrupt the blood-brain barrier and facilitate viral entry,21 and SARS-CoV-2 neurotropism, particularly that resulting in viral entry via olfactory and brainstem routes,22 was described early in the pandemic. In an animal model, administration of the S1 subunit of the SARS-CoV-2 spike protein into the CNS resulted in neurotoxicity mediated by IL-1β induction and microglial activation.23 It has further been shown that the spike protein specifically activates the NF-κB pathway, leading to the induction of proinflammatory cytokines,24 and this pathway is known to be associated with aggressive glioblastoma behavior.25,26 Interestingly, SARS-CoV-2–infected airway epithelial cells have been shown to have low expression of transcription factors that downregulate MAPK pathway signaling,27 suggesting that SARS-CoV-2 might have a direct oncogenic effect on the tumor environment that could have contributed to the aggressive cases that we observed. If so, this explanation could correspond with our observation that viral infection supported a permissive tumor environment, preceding new diagnoses in 7 cases at a short interval. Notably, we did not detect a difference in the prevalence of MAPK alterations between cases and controls.
Our analyses were limited by factors inherent to retrospective chart review, the heterogeneity of time points at which patients were tested for or developed COVID-19 during their glioblastoma disease course, and the evolution of virus biology and societal responses to the pandemic during the period that was analyzed. We attempted to reduce the effect of heterogeneous testing time points and the potential for lead-time bias by stratifying patients by when they underwent COVID-19 testing relative to their glioblastoma diagnosis. Several case patients were still alive when our dataset was locked, which, combined with the limitations of the sample size, may have obscured the OS analysis. Nevertheless, the trend of reduced OS among all cases was significant among Y1 cases and remained apparent but not significant among Y2 cases.
The effects of the COVID-19 mRNA vaccine on tumor biology and progression, particularly given that the leading mRNA vaccines encode for a modified spike protein, remain unclear. Interestingly, we captured a higher proportion of COVID-19 vaccine–exposed individuals in our cases than in our controls, but the difference was not significant, and there was no apparent correlation between the timing of the vaccine and the development of progression. Comparing pooled population vaccination data with Central Brain Tumor Registry (CBTRUS) reports could be a useful future exploration for elucidating a relationship.
On May 5, 2023, the World Health Organization announced that the global public health emergency due to COVID-19 was over due to reduced deaths and decreased pressure on healthcare systems.28 Nevertheless, despite reduced monitoring efforts, the virus is expected to remain impactful both directly and indirectly, given the excess mortality observed during the pandemic.29 Therefore, our practice is to continue advising our patients to exercise caution and avoid exposure as much as possible. For patients who develop symptoms of COVID-19, close observation for 1 to 2 months is recommended, potentially accompanied by follow-up with short-interval imaging. Future analyses could further investigate whether fluctuations in peripheral blood cell lineages occur before or after COVID-19 infection and in association with expected hematopoietic disturbances during glioblastoma therapy. Additionally, tumor sampling after COVID-19 infection and analysis of the immune microenvironment could potentially reveal generalizable insights into the conditions that favor tumor progression.
Acknowledgments
Supported by the NIH/NCI under award number P30CA016672 (used by the Institutional Review Board). We thank Madison Semro, Associate Scientific Editor, and Erica Goodoff, Senior Scientific Editor, in the Research Medical Library at The University of Texas MD Anderson Cancer Center, for editing this article.
Contributor Information
Timothy A Gregory, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA; Department of Neurology, Madigan Army Medical Center, Tacoma, Washington, USA.
Stephanie R Knight, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Ashley E Aaroe, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Kaitlin N Highsmith, Department of Neuro-Oncology, Pharmacy Clinical Programs, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Zachary C Janatpour, Department of Neurology, Madigan Army Medical Center, Tacoma, Washington, USA.
Barbara J O’Brien, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Nazanin K Majd, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Monica E Loghin, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Chirag B Patel, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Shiao-Pei Weathers, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Vinay K Puduvalli, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Carlos Kamiya-Matsuoka, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Conflict of interest statement
V.K.P. reports the following disclosures: Advisory board role and honoraria from Servier, Insightec, Novocure, and Orbus therapeutics, and equity in Moderna, Amarin and Gilead. The other authors have no conflicts of interest to disclose.
Funding
None declared.
Disclaimer
The views expressed in this manuscript are those of the authors and do not reflect the official views of Madigan Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of Defense, the Department of the Army, or the U.S. Government.
References
- 1. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Accessed March 15, 2023. https://coronavirus.jhu.edu/map.html [Google Scholar]
- 2. Tabrizi S, Trippa L, Cagney D, et al. A quantitative framework for modeling COVID-19 risk during adjuvant therapy using published randomized trials of glioblastoma in the elderly. Neuro Oncol. 2020;22(7):918–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mohile NA, Blakeley JO, Gatson NTN, et al. Urgent considerations for the neuro-oncologic treatment of patients with gliomas during the COVID-19 pandemic. Neuro Oncol. 2020;22(7):912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aaroe A, Majd N, O’Brien B, et al. ; D3CODE Team. COVD-30. A snapshot of the impact of COVID-19 on patients with nervous system tumors. Neuro-Oncology. 2020;22(Supplement_2):ii27–ii27. [Google Scholar]
- 5. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou Z, Kang H, Li S, Zhao X.. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol. 2020;267(8):2179–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uversky VN, Elrashdy F, Aljadawi A, et al. Severe acute respiratory syndrome coronavirus 2 infection reaches the human nervous system: How? J Neurosci Res. 2021;99(3):750–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kyriakopoulos AM, Nigh G, McCullough PA, Seneff S.. Mitogen activated protein kinase (MAPK) activation, p53, and autophagy inhibition characterize the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein induced neurotoxicity. Cureus. 2022;14(12):e32361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lei J, Liu Y, Xie T, et al. Evidence for residual SARS-CoV-2 in glioblastoma tissue of a convalescent patient. Neuroreport. 2021;32(9):771–775. [DOI] [PubMed] [Google Scholar]
- 10. Chen A, Zhao W, Li X, et al. Comprehensive oncogenic features of coronavirus receptors in glioblastoma multiforme. Front Immunol. 2022;13:840785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Junqueira C, Crespo A, Ranjbar S, et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature. 2022;606(7914):576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ryan FJ, Hope CM, Masavuli MG, et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. 2022;20(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leon J, Michelson DA, Olejnik J, et al. A virus-specific monocyte inflammatory phenotype is induced by SARS-CoV-2 at the immune-epithelial interface. Proc Natl Acad Sci USA. 2022;119(1):e2116853118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chukwueke UN, Wen PY.. Use of the response assessment in neuro-oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncology. 2019;8(1):CCNS28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lo Russo G, Moro M, Sommariva M, et al. Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin Cancer Res. 2019;25(3):989–999. [DOI] [PubMed] [Google Scholar]
- 16. Kaplan EL, Meier P.. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 17. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163–170. [PubMed] [Google Scholar]
- 18. Files JK, Boppana S, Perez MD, et al. Sustained cellular immune dysregulation in individuals recovering from SARS-CoV-2 infection. J Clin Invest. 2021;131(1):e140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wen W, Su W, Tang H, et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020;6(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bergamaschi L, Mescia F, Turner L, et al. ; Cambridge Institute of Therapeutic Immunology and Infectious Disease-National Institute of Health Research (CITIID-NIHR) COVID BioResource Collaboration. Longitudinal analysis reveals that delayed bystander CD8+ T cell activation and early immune pathology distinguish severe COVID-19 from mild disease. Immunity. 2021;54(6):1257–1275.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boroujeni ME, Simani L, Bluyssen HAR, et al. Inflammatory response leads to neuronal death in human post-mortem cerebral cortex in patients with COVID-19. ACS Chem Neurosci. 2021;12(12):2143–2150. [DOI] [PubMed] [Google Scholar]
- 22. Bulfamante G, Chiumello D, Canevini MP, et al. First ultrastructural autoptic findings of SARS-Cov-2 in olfactory pathways and brainstem. Minerva Anestesiol. 2020;86(6):678–679. [DOI] [PubMed] [Google Scholar]
- 23. Oh J, Cho WH, Barcelon E, et al. SARS-CoV-2 spike protein induces cognitive deficit and anxiety-like behavior in mouse via non-cell autonomous hippocampal neuronal death. Sci Rep. 2022;12(1):5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khan S, Shafiei MS, Longoria C, et al. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. Elife. 2021;10:e68563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McFarland BC, Hong SW, Rajbhandari R, et al. NF-κB-induced IL-6 ensures STAT3 activation and tumor aggressiveness in glioblastoma. PLoS One. 2013;8(11):e78728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zanotto-Filho A, Gonçalves RM, Klafke K, et al. Inflammatory landscape of human brain tumors reveals an NFκB dependent cytokine pathway associated with mesenchymal glioblastoma. Cancer Lett. 2017;390:176–187. [DOI] [PubMed] [Google Scholar]
- 27. Goel S, Saheb Sharif-Askari F, Saheb Sharif Askari N, et al. SARS-CoV-2 switches “on” MAPK and NFκB signaling via the reduction of nuclear DUSP1 and DUSP5 expression. Front Pharmacol. 2021;12:631879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lenharo M. WHO declares end to COVID-19’s emergency phase. Nature. 2023:d41586-023-01559-z. [DOI] [PubMed] [Google Scholar]
- 29. COVID-19 Excess Mortality Collaborators. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet. 2022;399(10334):1513–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]