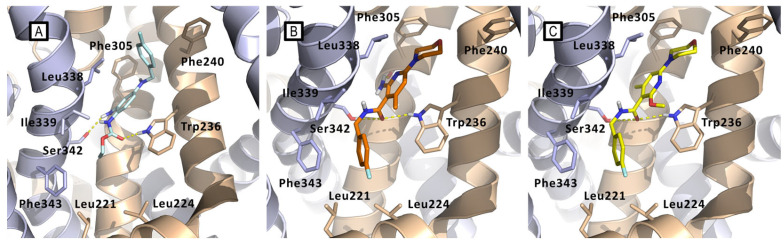
Figure 21.
Comparison between the docking-predicted Kv7.2/7.3-bound conformations of (A) RTG (cyan sticks); (B) compound 29 (orange sticks); (C) compound 26 (yellow sticks). Kv7.2/7.3 subunits are represented as wheat and azure cartoons, respectively. Main residues of the pocket are represented as sticks. All three compounds maintain the H-bond interaction with Trp236, showing only a slight displacement of the central pyridine ring in respect to the core phenyl ring of RTG. Ser342 makes H-bonds with the three compounds, but 26 and 29 make it by their carbonylic oxygen rather than with the amino group as in the case of RTG. The amino group of 29 is instead directed toward a hydrophobic spot, where even the methyl substituent of 26 is found. The 4-fluorobenzyl group of 26 and 29 lay in the large hydrophobic pocket formed by Leu221, Leu225, and Phe343.