Table 3.
Selective hydroxylation of benzene to phenol by some reported Fe-based heterostructures [149,151,152,153,154].
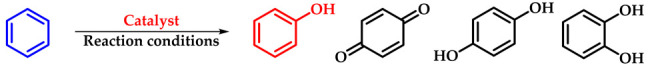
| |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Amount (mg) | Temp. (°C) | Time (h) | % Sel. (Phe.) |
| 1 | Fe-MFI-Zeolite | 100 | 60 | 2 | 97 |
| 2 | Fe-Cu/Beta Zeolite | 50 | 60 | 6 | 96 |
| 3 | Cu-Fe/SBA-15 | 30 | 60 | 3 | 93 |
| 4 | Fe-NC | 30 | 60 | 4 | 95 |
| 5 | CuO-Fe2O3/Fe3O4 | 50 | 40 | 24 | 85 |
All the reactions were performed in CH3CN solvent and H2O2 as oxidant, Temp. = Temperature, % Sel. (Phe.) means % Selectivity of phenol.
