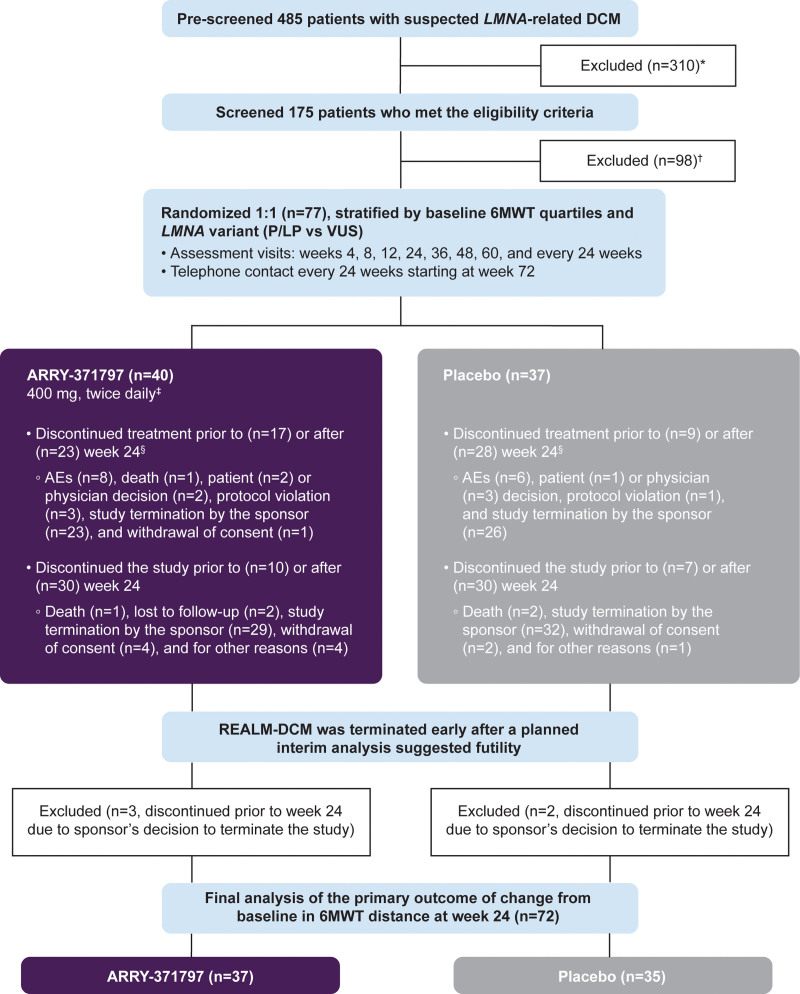
Figure 1.
Study design and patient disposition. 6MWT indicates 6-minute walk test; AE, adverse event; BID, twice daily; DCM, dilated cardiomyopathy; KCCQ, Kansas City Cardiomyopathy Questionnaire; LMNA, lamin A/C; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PL, physical limitation; and TS, total symptom. *Prescreen failure included patients who signed the prescreening informed consent but did not sign the main study informed consent. †Screen failure included patients who signed the prescreening and main study informed consent but were not subsequently randomized. ‡Dose reduction for safety or tolerability were allowed throughout this study. The ARRY-371797 400 mg BID dose could be reduced to 200 mg BID and subsequently to 100 mg BID if necessary. §Patients who discontinued treatment can stay in the study for additional follow-up.