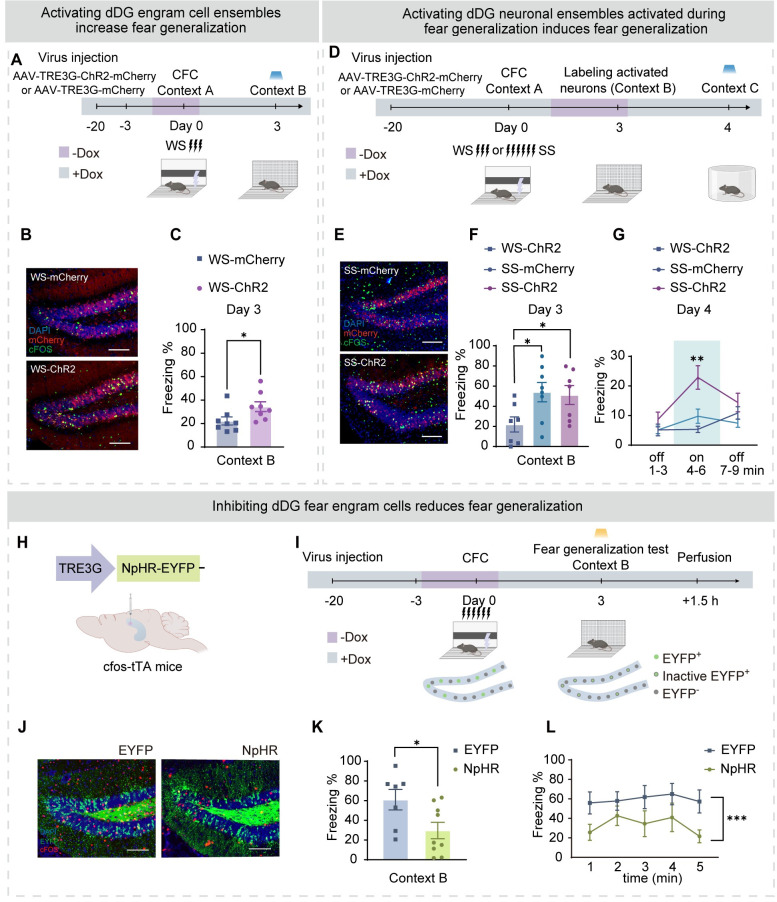
Fig 3. Activated dDG fear engram cells are both sufficient and necessary for fear generalization.
(A) Experimental design. (B) Representative images of mCherry+ expressing (red) and cFOS+ immunostaining (green) in the dDG. Top: WS-mCherry group. Bottom: SS-ChR2 group. Scale bar, 100 μm. (C) Compared with WS-mCherry group, WS-ChR2 group displayed a higher percentage of freezing levels during the fear generalization test (unpaired Student’s t test). (D) Experimental design. (E) Representative images of mCherry+ expressing (red) and cFOS+ immunostaining (green) in the dDG. Left: SS-mCherry group. Right: SS-ChR2 group. Scale bar, 100 μm. (F) The WS-ChR2 group showed a low level of freezing, while the SS-mCherry group and SS-ChR2 group showed a high level of freezing in context B (one-way ANOVA with Bonferroni’s post hoc test). (G) During laser presentation, the SS-ChR2 group showed a high level of freezing in context C (two-way ANOVA with Bonferroni’s post hoc test). (H) Optogenetic design. The dDG of cfos-tTA mice was injected with AAV9-TRE3G-EYFP (EYFP group) or AAV9-TRE3G-NpHR-EYFP (NpHR group) virus. The optic fiber was embedded above the dDG. (I) Experimental design. (J) Representative images of EYFP+ expression (green) and cFOS+ immunostaining (red) in the dDG. Left: EYFP group. Right: NPHR group. Scale bar, 100 μm. (K) The NPHR group displayed a lower percentage of freezing levels during the memory recall test (unpaired Student’s t test). (L) The NPHR group displayed a lower percentage of freezing levels during the fear generalization test from 1 min to 5 min (two-way ANOVA with Bonferroni’s post hoc test). In Fig 3, data were presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. The underlying data and statistical information in Fig 3 can be found in S1 Data. The mice depicted were created with BioRender.com. dDG, dorsal dentate gyrus; SS, strong shock; WS, weak shock.