Abstract
Purpose
Family history is one of the strongest risk factors for inflammatory bowel diseases (IBD) while studies about the clinical phenotype of familial IBD are limited. This study aimed to compare the phenotypic features of familial Crohn’s disease (CD) with sporadic CD.
Methods
Familial CD was defined as CD patients having one or more first, second, third, fourth degree, or above relatives with CD. Sporadic CD patients hospitalized during the same period were matched 1:3 by age and gender. Differences in clinical characteristics, phenotype distribution, extraintestinal manifestations, and complications at diagnosis, as well as treatment regimen and surgery, were compared between familial and sporadic CD.
Results
The familial CD was associated with a higher rate of past appendectomy history (P = 0.009), more intestinal perforation at onset (P = 0.012), more MRI results of anal lesion (P = 0.023), and gastrointestinal perforation (P = 0.040) at diagnosis, higher rate of past intestinal surgery history (P = 0.007), more number of intestinal surgeries (P = 0.037), longer duration of follow-up (P = 0.017), lower rate of taking biologicals for current maintenance (P = 0.043), lower tendency to upgrade to biologicals during follow-up (P = 0.013), higher possibility to experience gastrointestinal obstruction (P = 0.047), and abdominal abscess during follow-up (P = 0.045).
Conclusion
Familial CD is associated with a more aggressive clinical phenotype.
Keywords: Crohn’s disease, Familial, Sporadic, Phenotype
Introduction
Inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), are chronic and relapsing diseases that distress millions of people worldwide. During the last two decades, the number of IBD patients in Asia including China increased rapidly, attracting more and more attention from doctors [1]. The pathogenic mechanisms behind IBD are not yet fully defined, while the current hypothesis suggests that genetic variations, immunological alterations, shifts in the gut microbiome, and external environmental influences altogether affect disease development [2, 3]. Family history is one of the strongest risk factors for IBD [4–8].
Epidemiological surveys showed that there were great differences in the incidence of IBD among different ethnic groups. The incidence of IBD was the highest among Caucasians, followed by African Americans, and the lowest among Asians. A positive family history increases the risk 10–15 times for IBD development [5, 9, 10]. So we believe that genetic predisposition is a strong factor influencing the development of IBD.
Asian IBDs have shown great differences from Western IBDs, including the genetic factor. Polymorphisms in nucleotide-binding oligomerization domain 2 (NOD2) contribute the largest fraction of genetic risk for Western Crohn’s disease so far, while we found no mutation in the Chinese Han population [11]. A study from Denmark showed that up to 12% of all IBD cases have a positive family history [12]. An American study in 1996 reported the prevalence of familial IBD ranged between 10 and 25% [13]. While in Asians, we used to think that IBD is sporadic. As the incidence rises, family history is becoming more evident. The IBD patients with a family history take 1 to 6% proportions in Asian populations [5, 14–18].
Although many studies have confirmed the influence of family history on the risk of IBD disease, few studies have focused on whether family history influences the clinical features, disease course, and severity of patients with IBD. There is still controversy about the difference between sporadic and familial IBD. Some studies suggest that familial IBD has an earlier onset, higher disease severity, and is more aggressive [12, 19, 20], while others suggest that genetic influences are overestimated and do not show significant differences in clinical features between familial and sporadic IBD cases [7, 15, 21]. There is a lack of studies on familial IBD in Chinese populations, and it is worth exploring whether there are differences in the clinical features between familial and sporadic IBD. Moreover, most familial IBD studies have limited and ambiguous data. Consequently, we performed this study and obtained more accurate information by telephone follow-up. We aimed to provide a better understanding of the effect of familial CD on the clinical phenotype and disease course, which might be helpful for clinical decision-making.
Materials and methods
Study design
This hospital-based prospective study was performed at the Department of Gastroenterology, the Sir Run Run Shaw Hospital, Zhejiang University School of Medicine (Hangzhou, Zhejiang Province, China). The study protocol was approved by the Ethical Review Committee of the Sir Run Run Shaw Hospital (no. 20211103–36). Informed consent was obtained from each patient included in the study. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) as reflected in a priori approval by the institution’s human research committee.
Study population
This study included patients recruited in a prospective registry, which has been maintained since 2019.01.01 at the Sir Run Run Shaw Hospital and other hospitals. All adult patients aged 18 years and older with a confirmed diagnosis of CD, UC, or IBD-unspecified (IBDU), seeking care at the Inflammatory Bowel Disease Center of Sir Run Run Shaw Hospital, were eligible for inclusion in the cohort. All patients were followed up and data in this study ended at 2021.12.31.
A family history of IBD was assessed by a detailed questionnaire ascertaining the presence of either CD or UC in a first degree (parent, child, sibling), second degree (grandparent, uncle, aunt), or distant relative. Patients were considered to have familial IBD in the presence of CD or UC in any relative. Patients with no reported family history were considered to have sporadic IBD [4].
Data collection
Demographics and clinical characteristics including age, gender, growing environment before 14 years old, history of smoking, education, past medical history, breastfeeding time, age at onset, clinical characteristics at onset, course of disease, age at diagnosis, Montreal classification, perianal lesions, MRI results of anal, gastrointestinal tumor, gastrointestinal bleeding, gastrointestinal perforation, gastrointestinal obstruction, abdominal abscess, intestinal fistula, extraintestinal manifestations, age at the first intestinal surgery, resection of the intestine segments > 1 m, time between multiple surgeries, number of surgeries within 1 year of onset, number of surgeries within 2 year of onset, number of intestinal surgeries, history of intestinal surgery, causes of intestinal surgery, number of perianal surgeries, history of perianal surgery, causes of perianal surgery, number of endoscopic surgeries, history of endoscopic surgery, causes of endoscopic surgery, history of appendix surgery, duration of follow-up, current maintenance drug, upgrade to biologicalsals, type of biologicalsals, loss of response to biologicalsals, current disease location, current disease behavior, gastrointestinal tumor during follow-up, gastrointestinal bleeding during follow-up, gastrointestinal perforation during follow-up, gastrointestinal obstruction during follow-up, abdominal abscess during follow-up, intestinal fistula during follow-up were obtained and confirmed.
Statistical analysis
Continuous variables are presented as medians with interquartile ranges (IQRs), and categorical variables are presented as numbers with percentages. Two-tailed unpaired t-test or Mann–Whitney U test was used to compare continuous variables, and the χ2 test or the Fisher exact test was used to compare categorical variables, as appropriate. The cumulative rate of upgrade to biologicals was compared by survival analysis using the log-rank test. A P value < 0.05 was considered statistically significant. Statistical analysis was performed using R software version 4.2.
Results
Prevalence of familial IBD
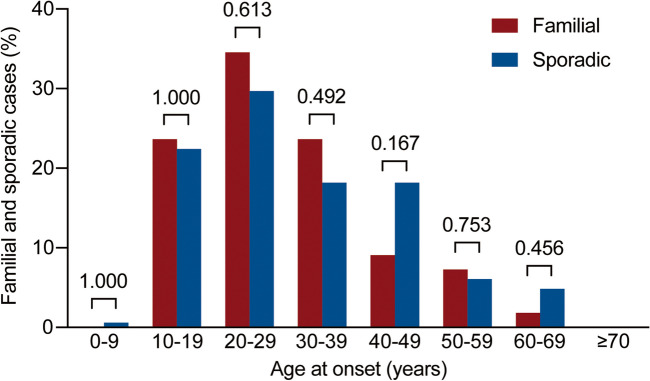
At the time of this study, 2493 IBD patients were included, with 2043 CD and 450 UC patients. Overall, 60 out of 2493 IBD patients (2.39%) had familial IBD, including 55 CD patients and 5 UC patients (Fig. 1). The prevalence of family history according to the age of onset is shown in Fig. 2. The number of cases for both familial and sporadic CD increased before 30 years of age with age and decreased after 30 years of age with age. The maximum number of cases for both familial and sporadic CD were in the 20–29-year age group.
Fig. 1.
Flow diagram showing the distribution of familial and sporadic IBD
Fig. 2.
Bar diagram showing familial and sporadic Crohn’s disease (CD) cases by age at onset
Demographic characteristics of familial CD and sporadic CD
Familial CD and sporadic CD were similar in age, gender, growing environment, history of smoking, education, and breastfeeding time, the comparison did not show significant differences. However, familial CD was associated with a higher percentage of past appendectomy history (20.00% vs 6.67%, P = 0.009) (Table 1).
Table 1.
Demographic characteristics of familial and sporadic Crohn’s disease (CD)
| Familial (N = 55) | Sporadic (N = 165) | P value | |
|---|---|---|---|
| Age, years (median [IQR]) | 35.00 [26.00; 48.50] | 35.00 [26.00; 50.00] | 0.982 |
| Gender, n (%) | 0.937 | ||
| Male | 32 (58.18) | 93 (56.36) | |
| Female | 23 (41.82) | 72 (43.64) | |
| Growing environment before 14 years old, n (%) | |||
| City | 6 (10.91) | 37 (24.03) | 0.061 |
| Town | 12 (21.82) | 19 (12.34) | 0.140 |
| Country | 36 (65.45) | 98 (63.64) | 0.938 |
| History of smoking, n (%) | |||
| No | 30 (54.55) | 83 (53.55) | 1.000 |
| Yes | 4 (7.27) | 15 (9.68) | 0.786 |
| Former smoker | 7 (12.73) | 20 (12.90) | 1.000 |
| Passive smoker | 14 (25.45) | 37 (23.87) | 0.958 |
| Education, n (%) | |||
| Illiteracy | 1 (1.82) | 2 (1.30) | 1.000 |
| Primary school | 6 (10.91) | 15 (9.74) | 1.000 |
| Junior high school | 12 (21.82) | 37 (24.03) | 0.884 |
| Senior high school | 11 (20.00) | 25 (16.23) | 0.669 |
| Technical college | 10 (18.18) | 26 (16.88) | 0.991 |
| University | 14 (25.45) | 45 (29.22) | 0.720 |
| Master | 0 (0.00) | 4 (2.60) | 0.575 |
| Doctor | 1 (1.82) | 0 (0.00) | 0.263 |
| Past medical history, n (%) | |||
| No | 39 (70.91) | 123 (74.55) | 0.724 |
| Appendectomy | 11 (20.00) | 11 (6.67) | 0.009* |
| Tonsillectomy | 1 (1.82) | 2 (1.21) | 1.000 |
| Infectious mononucleosis | 0 (0.00) | 0(0.00) | |
| Eczema | 5 (9.09) | 16 (9.70) | 1.000 |
| Asthma | 2 (3.64) | 1 (0.61) | 0.155 |
| Other autoimmune disorders | 1 (1.82) | 4 (2.42) | 1.000 |
| Breastfeeding time, n (%) | |||
| No | 7 (12.73) | 9 (5.45) | 0.129 |
| < 3 months | 0 (0.00) | 4 (2.42) | 0.574 |
| 3–6 months | 3 (5.45) | 10 (6.06) | 1.000 |
| 6–12 months | 19 (34.55) | 43 (26.06) | 0.299 |
| ≥ 12 months | 26 (47.27) | 76 (46.06) | 1.000 |
*Statistically significant results
Abbreviations; IQR interquartile range
Clinical characteristics at onset and diagnosis of familial CD and sporadic CD
Most clinical characteristics at onset were not significantly different between familial CD and sporadic CD, while intestinal perforation was more common in familial CD (12.73% vs 3.03%, P = 0.012) (Table 2).
Table 2.
Clinical characteristics at onset of familial and sporadic Crohn’s disease (CD)
| Familial (N = 55) | Sporadic (N = 165) | P value | |
|---|---|---|---|
| Age at onset, years (median [IQR]) | 27.00 [20.00; 37.00] | 29.00 [21.00; 41.00] | 0.436 |
| Clinical characteristics at onset, n (%) | |||
| Abdominal pain | 32 (58.18) | 108 (65.45) | 0.418 |
| Diarrhea | 22 (40.00) | 74 (44.85) | 0.638 |
| Bloody stool | 9 (16.36) | 25 (15.15) | 1.000 |
| Abdominal mass | 0 (0.00) | 1 (0.61) | 1.000 |
| Anemia | 0 (0.00) | 4 (2.42) | 0.574 |
| Fever | 6 (10.91) | 11 (6.67) | 0.380 |
| Weight loss | 19 (34.55) | 42 (25.45) | 0.258 |
| Extraintestinal manifestations at onset | 3 (5.45) | 19 (11.52) | 0.299 |
| Perianal lesions at onset | 16 (29.09) | 45 (27.27) | 0.931 |
| Intestinal obstruction | 5 (9.09) | 21 (12.73) | 0.630 |
| Intestinal perforation | 7 (12.73) | 5 (3.03) | 0.012* |
*Statistically significant results
Abbreviations: IQR, interquartile range
As for clinical characteristics at diagnosis, MRI results of anal lesion (85.45% vs 68.48%, P = 0.023) and gastrointestinal perforation (14.55% vs 5.45%, P = 0.040) were more common in familial CD. There was no difference in the course of the disease, age at diagnosis, Montreal classification (age, location, and behavior), perianal lesions, gastrointestinal tumor, gastrointestinal bleeding, gastrointestinal obstruction, abdominal abscess, and intestinal fistula (Table 3).
Table 3.
Clinical characteristics at diagnosis of familial and sporadic Crohn’s disease (CD)
| Familial (N = 55) | Sporadic (N = 165) | P value | |
|---|---|---|---|
| Course of disease, years (median [IQR]) | 6.00 [2.00; 21.50] | 11.00 [3.00; 28.00] | 0.142 |
| Age at diagnosis, years (median [IQR]) | 29.00 [21.50; 38.00] | 31.00 [23.00; 44.00] | 0.358 |
| Montreal classification age at diagnosis, n (%) | |||
| ≤ 16 years old | 4 (7.27) | 12 (7.27) | 1.000 |
| 17–40 years old | 39 (70.91) | 99 (60.00) | 0.198 |
| > 40 years old | 12 (21.82) | 54 (32.73) | 0.174 |
| Montreal classification-location, n (%) | |||
| L1 ileal | 16 (29.09) | 46 (27.88) | 1.000 |
| L2 colonic | 1 (1.82) | 9 (5.45) | 0.457 |
| L3 ileocolonic | 29 (52.73) | 75 (45.45) | 0.436 |
| L4 upper | 0 (0.00) | 1 (0.61) | 1.000 |
| L4 + L1 | 4 (7.27) | 13 (7.88) | 1.000 |
| L4 + L2 | 0 (0.00) | 0 (0.00) | |
| L4 + L3 | 5 (9.09) | 20 (12.12) | 0.713 |
| Montreal classification-behavior, n (%) | |||
| B1 non-structuring, non-penetrating | 29 (52.73) | 91 (55.15) | 0.876 |
| B2 stricturing | 14 (25.45) | 48 (29.09) | 0.729 |
| B3 penetrating | 4 (7.27) | 13 (7.88) | 1.000 |
| B2 + B3 | 8 (14.55) | 12 (7.27) | 0.176 |
| Perianal lesions, n (%) | 36 (65.45) | 113 (68.48) | 0.803 |
| MRI results of anal, n (%) | |||
| No lesion | 8 (14.55) | 52 (31.52) | 0.023* |
| Perianal inflammation | 6 (10.91) | 7 (4.24) | 0.095 |
| Anal fistula | 27 (49.09) | 97 (58.79) | 0.272 |
| Perianal abscess | 14 (25.45) | 39 (23.64) | 0.927 |
| Others | 1 (1.82) | 0 (0.00) | 0.250 |
| Gastrointestinal tumor, n (%) | 0 (0.00) | 1 (0.61) | 1.000 |
| Gastrointestinal bleeding, n (%) | |||
| No | 48 (87.27) | 152 (92.12) | 0.417 |
| Once | 6 (10.91) | 8 (4.85) | 0.120 |
| Twice | 0 (0.00) | 3 (1.82) | 0.575 |
| 3 times | 0 (0.00) | 1 (0.61) | 1.000 |
| > 3 times | 1 (1.82) | 1 (0.61) | 0.438 |
| Gastrointestinal perforation, n (%) | |||
| No | 47 (85.45) | 156 (94.55) | 0.040* |
| Once | 8 (14.55) | 9 (5.45) | 0.040* |
| Twice | 0 (0.00) | 0 (0.00) | |
| 3 times | 0 (0.00) | 0 (0.00) | |
| > 3 times | 0 (0.00) | 0 (0.00) | |
| Gastrointestinal obstruction, n (%) | |||
| No | 45 (81.82) | 128 (77.58) | 0.635 |
| Once | 10 (18.18) | 24 (14.55) | 0.667 |
| Twice | 0 (0.00) | 8 (4.85) | 0.206 |
| 3 times | 0 (0.00) | 1 (0.61) | 1.000 |
| > 3 times | 0 (0.00) | 4 (2.42) | 0.574 |
| Abdominal abscess, n (%) | |||
| No | 53 (96.36) | 160 (96.97) | 1.000 |
| Once | 2 (3.64) | 5 (3.03) | 1.000 |
| Twice | 0 (0.00) | 0 (0.00) | |
| 3 times | 0 (0.00) | 0 (0.00) | |
| > 3 times | 0 (0.00) | 0 (0.00) | |
| Intestinal fistula, n (%) | |||
| No | 53 (96.36) | 154 (93.33) | 0.526 |
| Once | 2 (3.64) | 11 (6.67) | 0.526 |
| Twice | 0 (0.00) | 0 (0.00) | |
| 3 times | 0 (0.00) | 0 (0.00) | |
| > 3 times | 0 (0.00) | 0 (0.00) |
*Statistically significant results
Abbreviations: IQR interquartile range, MRI magnetic resonance imaging
Extraintestinal manifestations and surgical characteristics of familial CD and sporadic CD
Extraintestinal manifestations including peripheral arthritis/spondyloarthritis, pyoderma gangrenosum/aphthous ulcer/erythema nodosum/psoriasis, and iriditis/pigmentitis did not differ between familial CD and sporadic CD (Table 4).
Table 4.
Extraintestinal manifestations of familial and sporadic Crohn’s disease (CD)
| Familial (N = 55) | Sporadic (N = 165) | P value | |
|---|---|---|---|
| Extraintestinal manifestations, n (%) | 13 (23.64) | 46 (27.88) | 0.660 |
| Peripheral arthritis/spondyloarthritis, n (%) | 6 (10.91) | 18 (10.91) | 1.000 |
| Pyoderma gangrenosum/aphthous ulcer/erythema nodosum/psoriasis, n (%) | 8 (14.55) | 32 (19.39) | 0.545 |
| Iriditis/pigmentitis, n (%) | 1 (1.82) | 2 (1.21) | 1.000 |
As for surgical characteristics, familial CD were more likely to have intestinal surgery history and have more times of intestinal surgery each patient. The number of intestinal surgeries was larger in familial CD (1.50[IQR 1.00–2.00] vs 1.00[IQR 1.00–2.00], P = 0.037) and history of intestinal surgery was more common in familial CD (50.91% vs 29.70%, P = 0.007). Besides, the proportion of patients who undergo surgery due to obstruction (32.73% vs 16.36%, P = 0.016), perforation (18.18% vs 7.27%, P = 0.038), and other reasons (12.73% vs 1.82%, P = 0.003) was significantly higher among the familial CD. Furthermore, familial CD had a higher percentage of the history of appendix surgery (38.18% vs 14.55%, P < 0.001) (Table 5).
Table 5.
Surgical characteristics of familial and sporadic Crohn’s disease (CD)
| Familial (N = 55) | Sporadic (N = 165) | P value | |
|---|---|---|---|
| Age at the first intestinal surgery, years (median [IQR]) | 37.50 [31.50; 43.25] | 36.00 [26.00; 49.00] | 0.771 |
| Resection of the intestine segments > 1 m, n (%) | 7 (25.00) | 4 (8.16) | 0.086 |
| Time between multiple surgeries, months (median [IQR]) | 59.00 [8.75; 105.75] | 71.00 [14.25; 103.50] | 0.318 |
| Number of surgeries within 1 year of onset, n (%) | 11 (39.29) | 20 (40.82) | 1.000 |
| Number of surgeries within 2 years of onset, n (%) | 12 (42.86) | 26 (53.06) | 0.532 |
| Number of intestinal surgeries, n (median [IQR]) | 1.50 [1.00; 2.00] | 1.00 [1.00; 1.00] | 0.037* |
| History of intestinal surgery, n (%) | 28 (50.91) | 49 (29.70) | 0.007* |
| Causes of intestinal surgery, n (%) | |||
| Obstruction | 18 (32.73) | 27 (16.36) | 0.016* |
| Perforation | 10 (18.18) | 12 (7.27) | 0.038* |
| Intestinal fistula | 7 (12.73) | 17 (10.30) | 0.803 |
| Abdominal abscess | 3 (5.45) | 2 (1.21) | 0.101 |
| Canceration | 1 (1.82) | 0 (0.00) | 0.250 |
| Others | 7 (12.73) | 3 (1.82) | 0.003* |
| Number of perianal surgeries, n (median [IQR]) | 1.00 [1.00; 2.00] | 1.00 [1.00; 1.00] | 0.067 |
| History of perianal surgery, n (%) | 21 (38.18) | 54 (32.73) | 0.565 |
| Causes of perianal surgery, n (%) | |||
| Anal fistula | 13 (23.64) | 32 (19.39) | 0.629 |
| Perianal abscess | 15 (27.27) | 33 (20.00) | 0.346 |
| Others | 2 (3.64) | 3 (1.82) | 0.601 |
| Number of endoscopic surgeries, n (median [IQR]) | 1.00 [1.00; 1.00] | 1.00 [1.00; 1.00] | 0.564 |
| History of endoscopic surgery, n (%) | 2 (3.64) | 7 (4.24) | 1.000 |
| Causes of endoscopic surgery, n (%) | |||
| Stenosis dilation | 1 (1.82) | 6 (3.64) | 0.683 |
| Canceration | 0 (0.00) | 0 (0.00) | |
| Others | 1 (1.82) | 1 (0.61) | 0.438 |
| History of appendix surgery, n (%) | 21 (38.18) | 24 (14.55) | < 0.001* |
*Statistically significant results
Abbreviations: IQR interquartile range, IBD inflammatory bowel disease
Follow-up of familial CD and sporadic CD
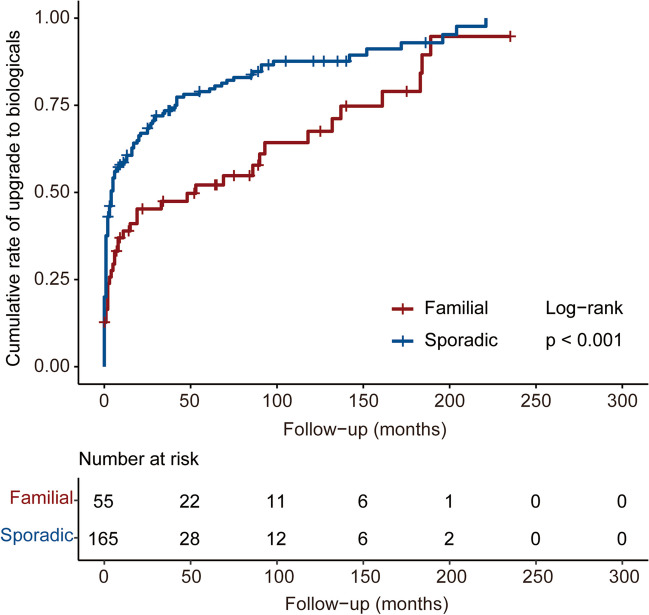
The median duration of follow-up in familial and sporadic CD patients was 54.00 months [IQR 21.50–125 months] and 41.00 months [IQR 14.00–79.00 months], respectively (P = 0.017). For the current maintenance drug, the percentage of patients taking 5-aminosalicylic (5-ASA) was significantly higher among the familial CD (9.09% vs 0.61%, P = 0.004), while the percentage of patients taking biologicals was significantly lower among the familial CD (61.82% vs 76.97%, P = 0.043). During follow-up, the familial CD was associated with a lower tendency to upgrade to biologicals (69.09% vs 85.37%, P = 0.013) (Table 6). The cumulative rate of upgrade to biologicals was also lower in familial CD (P < 0.001) (Fig. 3). During follow-up, the familial CD was associated with an increased possibility of experiencing gastrointestinal obstruction (23.64% vs 11.52%, P = 0.047) and abdominal abscess (9.09% vs 2.42%, P = 0.045). For current disease behavior, familial CD had a higher percentage of structuring and penetrating behavior (29.09% vs 13.94%, P = 0.019) (Table 6).
Table 6.
Follow-up of familial and sporadic Crohn’s disease (CD)
| Familial (N = 55) | Sporadic (N = 165) | P value | |
|---|---|---|---|
| Duration of follow-up, n (median [IQR]) | 54.00 [21.50;125.00] | 41.00 [14.00;79.00] | 0.017* |
| Current maintenance drug, n (%) | |||
| No | 6 (10.91) | 14 (8.48) | 0.787 |
| 5-ASA | 5 (9.09) | 1 (0.61) | 0.004* |
| Steroid | 2 (3.64) | 3 (1.82) | 0.601 |
| Immunosuppressant | 15 (27.27) | 26 (15.76) | 0.089 |
| Biologicals | 34 (61.82) | 127 (76.97) | 0.043* |
| Enteral nutrition | 3 (5.45) | 5 (3.03) | 0.416 |
| Clinical trial | 0 (0.00) | 2 (1.21) | 1.000 |
| Upgrade to biologicals, n (%) | 38 (69.09) | 140 (85.37) | 0.013* |
| Type of biologicals, n (%) | |||
| Infliximab | 33 (60.00) | 121 (73.33) | 0.089 |
| Adalimumab | 1 (1.82) | 4 (2.42) | 1.000 |
| Vedolizumab | 1 (1.82) | 2 (1.21) | 1.000 |
| Ustekinumab | 3 (5.45) | 10 (6.06) | 1.000 |
| Risankizumab | 0 (0.00) | 1 (0.61) | 1.000 |
| Clinical trial | 0 (0.00) | 2 (1.21) | 1.000 |
| Loss of response to biologicals, n (%) | 7 (18.42) | 14 (10.22) | 0.169 |
| Current disease location, n (%) | |||
| L1 ileal | 15 (27.27) | 45 (27.27) | 1.000 |
| L2 colonic | 1 (1.82) | 9 (5.45) | 0.457 |
| L3 ileocolonic | 29 (52.73) | 74 (44.85) | 0.391 |
| L4 upper | 0 (0.00) | 1 (0.61) | 1.000 |
| L4 + L1 | 4 (7.27) | 12 (7.27) | 1.000 |
| L4 + L2 | 0 (0.00) | 0 (0.00) | |
| L4 + L3 | 6 (10.91) | 24 (14.55) | 0.650 |
| Disease location change, n (%) | 2 (3.64) | 3 (1.82) | 0.601 |
| Current disease behavior, n (%) | |||
| B1 non-stricturing, non-penetrating | 19 (34.55) | 75 (45.45) | 0.208 |
| B2 stricturing | 14 (25.45) | 52 (31.52) | 0.497 |
| B3 penetrating | 6 (10.91) | 15 (9.09) | 0.895 |
| B2 + B3 | 16 (29.09) | 23 (13.94) | 0.019* |
| Disease behavior progression, n (%) | 15 (27.27) | 25 (15.15) | 0.069 |
| Gastrointestinal tumor, n (%) | 1 (1.82) | 0 (0.00) | 0.250 |
| Gastrointestinal bleeding, n (%) | |||
| No | 52 (94.55) | 158 (95.76) | 0.714 |
| Once | 3 (5.45) | 2 (1.21) | 0.101 |
| Twice | 0 (0.00) | 2 (1.21) | 1.000 |
| 3 times | 0 (0.00) | 1 (0.61) | 1.000 |
| > 3 times | 0 (0.00) | 2 (1.21) | 1.000 |
| Gastrointestinal perforation, n (%) | |||
| No | 51 (92.73) | 161 (97.58) | 0.110 |
| Once | 4 (7.27) | 4 (2.42) | 0.110 |
| Twice | 0 (0.00) | 0 (0.00) | |
| 3 times | 0 (0.00) | 0 (0.00) | |
| > 3 times | 0 (0.00) | 0 (0.00) | |
| Gastrointestinal obstruction, n (%) | |||
| No | 42 (76.36) | 146 (88.48) | 0.047* |
| Once | 9 (16.36) | 12 (7.27) | 0.085 |
| Twice | 1 (1.82) | 2 (1.21) | 1.000 |
| 3 times | 1 (1.82) | 4 (2.42) | 1.000 |
| > 3 times | 2 (3.64) | 1 (0.61) | 0.155 |
| Abdominal abscess, n (%) | |||
| No | 50 (90.91) | 161 (97.58) | 0.045* |
| Once | 5 (9.09) | 4 (2.42) | 0.045* |
| Twice | 0 (0.00) | 0 (0.00) | |
| 3 times | 0 (0.00) | 0 (0.00) | |
| > 3 times | 0 (0.00) | 0 (0.00) | |
| Intestinal fistula, n (%) | |||
| No | 46 (83.64) | 149 (90.30) | 0.270 |
| Once | 8 (14.55) | 16 (9.70) | 0.454 |
| Twice | 1 (1.82) | 0 (0.00) | 0.250 |
| 3 times | 0 (0.00) | 0 (0.00) | |
| > 3 times | 0 (0.00) | 0 (0.00) |
*Statistically significant results
Abbreviations: IQR interquartile range, 5-ASA 5-aminosalicylic acid
Fig. 3.
Cumulative rate of upgrade to biologicals in familial and sporadic Crohn’s disease (CD)
Phenotypic concordance among 43 pairs of familial CD
Twenty-nine out of the 43 pairs (67.44%) of familial CD were concordant for age at onset ≤ 40 years old. Concordance of disease location, disease behavior, and perianal lesions at diagnosis was 60.47%, 44.19%, and 60.47%, respectively. Upgrading to biologicals occurred in 25 of the 43 pairs (58.14%). For current disease location and behavior, concordance was 55.81% and 27.91%. The concordance was 51.16% for the history of intestinal surgery (Table 7).
Table 7.
Phenotypic concordance among 43 pairs of familial Crohn’s disease (CD)
| Concordant pairs | Concordance (%) | |
|---|---|---|
| Age at onset ≤ 40 years old | 29 | 67.44 |
| Disease location at diagnosis | 26 | 60.47 |
| Disease behavior at diagnosis | 19 | 44.19 |
| Perianal lesions at diagnosis | 26 | 60.47 |
| Upgrade to biologicals | 25 | 58.14 |
| Current disease location | 24 | 55.81 |
| Current disease behavior | 12 | 27.91 |
| History of intestinal surgery | 22 | 51.16 |
Discussion
This study describes the prevalence, demographic characteristics, clinical characteristics, extraintestinal manifestations, surgical characteristics, follow-up, and phenotypic concordance of familial CD and sporadic CD. The results indicated that familial CD was associated with a more aggressive clinical phenotype, such as more intestinal perforation at onset, more MRI results of the anal lesion at diagnosis, more likelihood of intestinal surgery history, more times of intestinal surgery, and more gastrointestinal obstruction and abdominal abscess during follow-up.
In terms of age, previous studies have found that familial IBD cases have an earlier age of onset and diagnosis [4, 22, 23]; however, this study did not find significant differences in age of onset or diagnosis between familial and sporadic cases, which may be due to the small sample size.
As for extraintestinal manifestations, there was no difference between familial and sporadic CD in our study, which is similar to the findings of Saberzadeh-Ardestani et al. and Chung et al. [7, 15]. However, in other studies, familial IBD was associated with 1.8–5.6 times higher extraintestinal manifestations [24, 25]. This variation may be attributed to the genetic and environmental diversity among different geographic areas.
Our major outcome is that familial CD shows a more aggressive clinical phenotype and needs more surgery. However, the literature about the severity of familial CD is controversial. Previous studies found no effect of family history on disease complications and need for surgery, including a small study of 17 Korean familial CD [15], a study of 181 Jewish CD [26], a cohort of Norway patients [27], a cohort of Finnish patients [28], and a large cohort of 240 Iranian familial CD [7]. In contrast to these findings and consistent with our observations, several studies have shown that familial CD has a higher proportion of perianal disease and penetrating behavior [22], is more aggressive [6], and has a higher prevalence of fistulizing disease [5]. This is in accordance with our finding as we found that familial CD had more intestinal perforations at onset, more MRI results of anal lesion at diagnosis, and more gastrointestinal perforation at diagnosis. As for surgical characteristics, we found that familial CD were more likely to have intestinal surgery history, have more times of intestinal surgery, and have more likelihood to undergo surgery due to obstruction and perforation, which correspond to a more aggressive clinical phenotype.
In terms of follow-up, this study found that the median follow-up time was significantly longer in familial CD than in sporadic CD. One possible explanation is that familial CD manifests as a phenotypically aggressive condition, necessitating regular follow-ups and intensive medical care, which consequently leads to better treatment adherence. As for maintenance drugs, most studies indicated that the rate of biological use was higher in familial CD [5, 23, 29], and some studies showed no difference [4, 30]. However, in this study, familial CD patients were associated with lower rates of escalation to biologicals and current biologicals use for maintenance of remission compared with sporadic CD. One possible reason is that familial CD patients have better treatment willingness and compliance, receive clinical intervention earlier, and achieve better disease control, thus less requiring biologicals. Another potential explanation is that familial IBD patients have higher surgery rates, and after surgical resection resulting in disease control, they did not need biologicals for maintenance but rather maintained remission using traditional 5-ASA and immunosuppressants instead. On the other hand, the affordability issues surrounding biologics, coupled with the easy availability and lower cost of immunomodulators in this context, could result in the use of less advanced therapies for familial CD.
Although our study demonstrated several differences in clinical phenotype between familial CD and sporadic CD, some limitations remain to be addressed. First, this study was mainly done at an IBD center of a tertiary hospital. The disease conditions tend to be more complex and severe, leading to selection bias that may not reflect the real study population. Second, this study was performed using the hospital-based registry and was not a population-based cohort study. Therefore, a population-based cohort study will be needed in the future. Third, the small sample size, short follow-up period, and lack of inclusion of more objective data such as laboratory tests, imaging, endoscopy, and pathology, reduce the reliability of the results. Larger and multi-center cohorts are essential to more robustly define the similarities and differences between familial and sporadic IBD. In addition, this study did not investigate genetic aspects of familial CD, which could provide crucial insights into inheritance patterns and disease mechanisms.
In conclusion, this study provides evidence of a more aggressive clinical phenotype in patients with familial CD compared with sporadic CD. These findings have important implications for clinical practice and would help the clinician to predict the course of the disease, stratify familial IBD as high risk, and initiate treatment accordingly.
Acknowledgements
We are grateful to our collaborators in the Department of Gastroenterology, Sir Run Run Shaw Hospital.
Author contribution
Conceptualization: QC; methodology: YZ and SD; writing—original draft: YZ and SD; writing—review and editing: YZ and XX; visualization: YZ and SD; supervision: LY and QC; funding acquisition: YZ, RL, and QC.
Funding
This study was supported by the National Natural Science Foundation of China (32000628), the Natural Science Foundation of Zhejiang Province (LQ21H030010 and LQ21H030013), and the Zhejiang Province Medicine and Health Research Project (2023KY805).
Data availability
Data will be made available on request.
Declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethical Review Committee of the Sir Run Run Shaw Hospital (no. 20211103–36). All patients gave their informed consent before their inclusion in the study. This article does not contain any studies with animals performed by any of the authors.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Patients signed informed consent regarding publishing their data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Siyuan Dong, Xiaoxia Xiang and Yu Zhang contributed equally.
References
- 1.Ng WK, Wong SH, Ng SC (2016) Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res 14(2):111–119. 10.5217/ir.2016.14.2.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres J, Mehandru S (2016) Jean-Frédéric Colombel LP-B. Crohn’s Disease The Lancet S0140–6736(16):31711–31721 [Google Scholar]
- 3.Ungaro R, Mehandru S, Allen PB et al (2017) Ulcerative colitis. Lancet 389(10080):1756–1770. 10.1016/S0140-6736(16)32126-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borren NZ, Conway G, Garber JJ et al (2018) Differences in clinical course, genetics, and the microbiome between familial and sporadic inflammatory bowel diseases. J Crohn’s Colitis 12(5):525–531. 10.1093/ecco-jcc/jjx154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee R, Pal P, Hutfless S et al (2019) Familial aggregation of inflammatory bowel disease in India: prevalence, risks and impact on disease behavior. Intest Res 17(4):486–495. 10.5217/ir.2018.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang SW, Kwak MS, Kim WS et al (2016) Influence of a positive family history on the clinical course of inflammatory bowel disease. J Crohn’s Colitis 10(9):1024–1032. 10.1093/ecco-jcc/jjw063 [DOI] [PubMed] [Google Scholar]
- 7.Saberzadeh-Ardestani B, Anushiravani A, Mansour-Ghanaei F et al (2022) Clinical phenotype and disease course of inflammatory bowel disease: a comparison between sporadic and familial cases. Inflamm Bowel Dis 28(7):1004–1011. 10.1093/ibd/izab202 [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Shah SC, Hann HJ et al (2021) Familial risk of inflammatory bowel disease: a population-based cohort study in South Korea. Clin Gastroenterol Hepatol 19(10):2128-2137.e15. 10.1016/j.cgh.2020.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annese V, Andreoli A, Astegiano M et al (2001) Clinical features in familial cases of Crohn’s disease and ulcerative colitis in Italy: a GISC study. Italian Study Group for the Disease of Colon and Rectum. Am. J. Gastroenterol. 96(10):2939–2945. 10.1111/J.1572-0241.2001.04685.X [DOI] [PubMed] [Google Scholar]
- 10.Probert CSJ, Jayanthi V, Hughes AO et al (1993) Prevalence and family risk of ulcerative colitis and Crohn’s disease: an epidemiological study among Europeans and South Asians in Leicestershire. Gut 34(11):1547–1551. 10.1136/GUT.34.11.1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao M, Cao Q, Luo LH et al (2005) NOD2/CARD15 Gene Polymorphisms and Susceptibility to Crohn’s Disease in Chinese Han Population. Zhonghua nei ke za zhi 44(3):210–212 [PubMed] [Google Scholar]
- 12.Moller FT, Andersen V, Wohlfahrt J et al (2015) Familial risk of inflammatory bowel disease: a population-based cohort study 1977–2011. Am J Gastroenterol 110(4):564–571. 10.1038/ajg.2015.50 [DOI] [PubMed] [Google Scholar]
- 13.Bayless TM, Tokayer AZ, Polito JM et al (1996) Crohn’s disease: concordance for site and clinical type in affected family members - potential hereditary influences. Gastroenterology 111(3):573–579. 10.1053/gast.1996.v111.pm8780559 [DOI] [PubMed] [Google Scholar]
- 14.Kuwahara E, Asakura K, Nishiwaki Y et al (2012) Effects of family history on inflammatory bowel disease characteristics in Japanese patients. J Gastroenterol 47(9):961–968. 10.1007/s00535-012-0558-3 [DOI] [PubMed] [Google Scholar]
- 15.Chung SH, Park SJ, Lee HS et al (2014) Similar clinical characteristics of familial and sporadic inflammatory bowel disease in South Korea. World J Gastroenterol 20(45):17120–17126. 10.3748/wjg.v20.i45.17120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang XL, Cui HF (2002) An analysis of 10218 ulcerative colitis cases in China. World J Gastroenterol 8(1):158–161. 10.3748/wjg.v8.i1.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang PQ, Hu J, Al Kazzi ES et al (2016) Family history and disease outcomes in patients with Crohn’s disease: a comparison between China and the United States. World J Gastrointest Pharmacol Ther. 7(4):556. 10.4292/WJGPT.V7.I4.556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng SC, Tang W, Ching JY et al (2013) Incidence and phenotype of inflammatory bowel disease based on results from the Asia-Pacific Crohn’s and colitis epidemiology study. Gastroenterology 145(1):158-165.e2. 10.1053/j.gastro.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 19.Santos MPC, Gomes C, Torres J (2018) Familial and ethnic risk in inflammatory bowel disease. Ann Gastroenterol 31(1):14–23. 10.20524/aog.2017.0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu S, Zou H, Zhang H et al (2018) Investigation of inflammatory bowel disease risk factors in 4 families in central China. Exp Ther Med 15(2):1367–1375. 10.3892/etm.2017.5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nunes T, Fiorino G, Danese S et al (2011) Familial aggregation in inflammatory bowel disease: is it genes or environment? World J Gastroenterol 17(22):2715–2722. 10.3748/wjg.v17.i22.2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreu M, Márquez L, Domènech E et al (2014) Disease severity in familial cases of IBD. J Crohn’s Colitis 8(3):234–239. 10.1016/j.crohns.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 23.Boaz E, Bar-gil A, Shitrit, Schechter M et al (2022) Inflammatory bowel disease in families with four or more affected first-degree relatives. Scand J Gastroenterol 1:5. 10.1080/00365521.2022.2106153 [DOI] [PubMed] [Google Scholar]
- 24.Vavricka SR, Brun L, Ballabeni P et al (2011) Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol 106(1):110–119. 10.1038/ajg.2010.343 [DOI] [PubMed] [Google Scholar]
- 25.Kayar Y, Dertli R, Konur S et al (2021) The development of extraintestinal manifestation and related risk factors in Crohn’s patients. Ir J Med Sci 190(2):597–604. 10.1007/s11845-020-02326-z [DOI] [PubMed] [Google Scholar]
- 26.Ben-Horin S, Avidan B, Yanai H et al (2009) Familial clustering of Crohn’s disease in Israel: prevalence and association with disease severity. Inflamm Bowel Dis 15(2):171–175. 10.1002/IBD.20740 [DOI] [PubMed] [Google Scholar]
- 27.Henriksen M, Jahnsen J, Lygren I et al (2007) Are there any differences in phenotype or disease course between familial and sporadic cases of inflammatory bowel disease? Results of a Population-Based Follow-up Study. Am J Gastroenterol 102(9):1955–1963. 10.1111/j.1572-0241.2007.01368.x [DOI] [PubMed] [Google Scholar]
- 28.Halme L, Turunen U, Heliö T et al (2002) Familial and sporadic in ammatory bowel disease. Scand J Gastroenterol 37(6):692–698 [DOI] [PubMed] [Google Scholar]
- 29.Ballester MP, Martí D, Tosca J et al (2017) Disease severity and treatment requirements in familial inflammatory bowel disease. Int J Colorectal Dis 32(8):1197–1205. 10.1007/S00384-017-2791-Y [DOI] [PubMed] [Google Scholar]
- 30.Chao CY, Bessissow T (2018) Does familial IBD have its own signature? J Crohn’s Colitis 12(5):515–516. 10.1093/ecco-jcc/jjy016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.