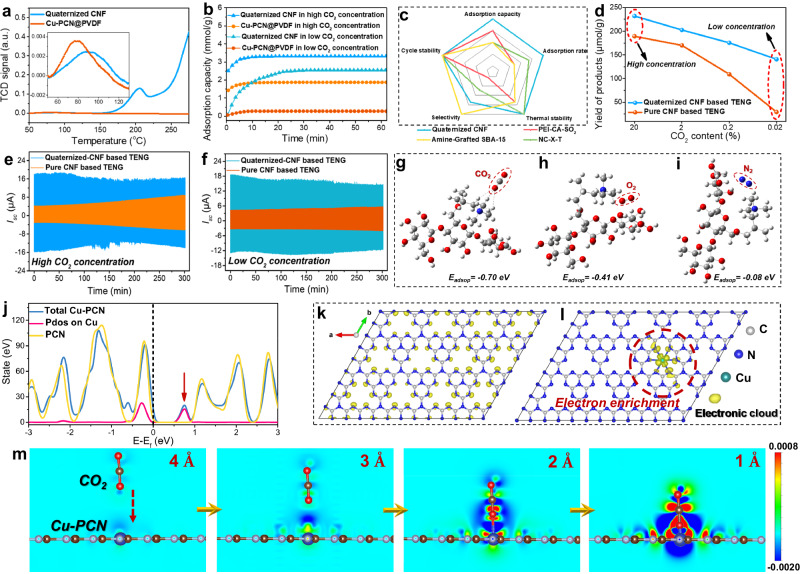
Fig. 3. Mechanism of contact-electro-catalytic CO2RR.
a Comparison of CO2-temperature programmed desorption (TPD) between quaternized CNF and Cu-PCN@PVDF. The inset is an amplification of the TCD signal in the range of 55 °C−125 °C. b Comparison of CO2 vapor adsorption at room temperature and pressure of quaternized CNF and Cu-PCN@PVDF with high and low CO2 concentrations. c A performance comparison of quaternized CNF with other materials that adsorb CO2. d Comparison of product yields of quaternized CNF-based TENG and pure CNF-based TENG under different CO2 concentrations for contact-electro-catalytic CO2RR. e, f Comparison of the current change of contact-electro-catalytic CO2RR of quaternary ammoniated CNF-based TENG and pure CNF-based TENG under high and low CO2 concentrations. g–i Comparison of adsorption energies of quaternized CNF for CO2, O2 and N2 molecules. j Analysis of the total density of states (TDOS) of Cu-PCN, projected density of states (PDOS) on Cu and the TDOS on PCN. k, l The electron distribution of the conduction band edge of PCN and Cu-PCN (iso surface = 0.004). m Charge distribution near Cu-PCN surface during contact. Source data are provided as a Source Data file.