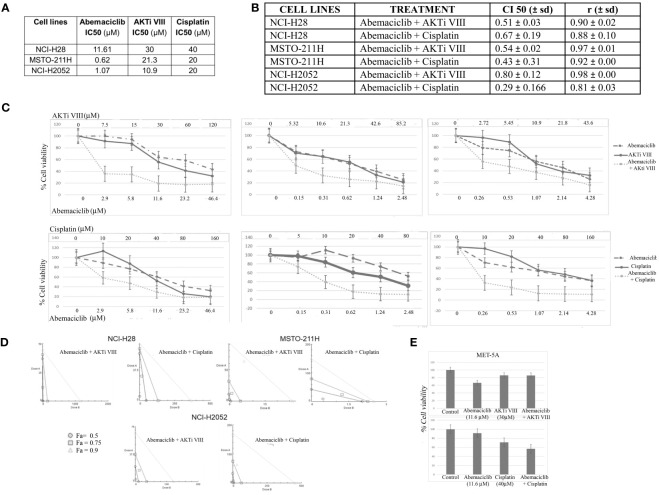
Figure 6.
Synergistic effects of abemaciclib/AKTi VIII and abemaciclib/cisplatin combination on DPM cell lines. (A) Abemaciclib, AKTi VIII, and cisplatin IC50 values on DPM cell lines. These values were calculated from cell viability data obtained by MTS after 72 h of treatment with three compounds. (B) Table reporting the means ± standard deviations of combination index (CI) and r values of abemaciclib–AKTi VIII and abemaciclib–cisplatin combination at 50% of cell killing (CI50) following 72 h of treatment, calculated using the CalcuSyn software for each of two independent experiments. (C) Dose–response curves for abemaciclib alone, AKTi VIII, cisplatin alone, and combinations in NCI-H28, MSTO-211H, and NCI-H2052 cell lines at 72 h after treatment. Results represent the means of two independent experiments in triplicate and are expressed as percentages of cell viability over control cells treated with DMSO alone. (D) Isobologram analysis to assess synergism between abemaciclib–AKTi VIII and abemaciclib–cisplatin. Isobolograms are derived from the mean values of the dose–response experiments reported in panel C, through the CompuSyn software at fixed effect levels [fraction affected (Fa)] of 50%, 75%, and 90%. The points below the lines indicate synergism. (E) Histogram showing that 72 h of treatment with abemaciclib–AKTi VIII and abemaciclib–cisplatin at indicated combination doses had no toxic effect on immortalized normal mesothelial cells (MET-5A), as determined by MTS assay. Results are reported as means of two independent experiments and expressed as percentages of cell viability compared to control cells treated with DMSO alone. DPM, diffuse pleural mesothelioma; DMSO, dimethyl sulfoxide.