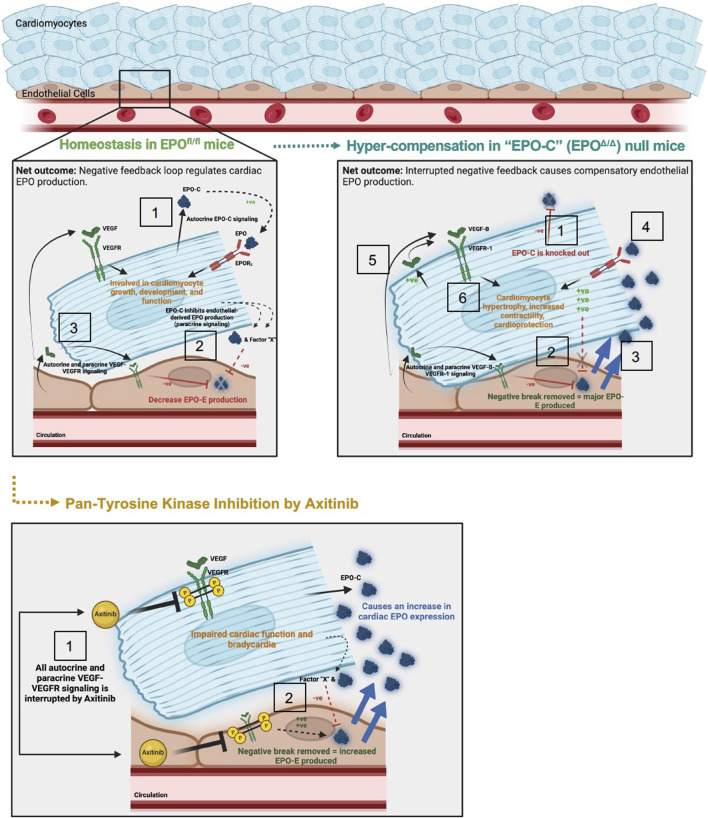
FIGURE 9.
Schematic of proposed mechanism: cardiac structure and function rely on the homeostatic interaction between EPO-EPOR and VEGF-VEGFR signaling. Top left panel: (1) In adult wildtype mice (EPOfl/fl), the cardiomyocyte produces low levels of Epo at baseline (Figure 1). (2) Cardiomyocyte derived EPO (“EPO-C″) represses endothelial cell EPO (“EPO-E″) production in a paracrine fashion. (3) VEGF elicits paracrine stimulation of VEGFR on neighboring endothelial cells and cardiomyocytes, and activation of this pathway inhibits EPO-E production. The net outcome of these stimuli is reciprocal endothelial repression of EPO production. Top right panel: (1) In EPO-C null mice (EPOΔ/Δ) EPO is successfully knocked out of the cardiomyocyte. (2) The lack of EPO produced by the cardiomyocytes releases the inhibition of EPO-EPOR by the endothelial cell in a paracrine fashion. (3) This leads to overproduction of EPO by the endothelial cell. (4) The overproduction of EPO positively feeds back and binds the EPOR2 located on the cardiomyocyte (Wright et al., 2004). EPO binding to the EPOR2 on the cardiomyocyte may induce cardiomyocyte-production of VEGF. (5) Increased VEGF-B-VEGFR-1 signaling, along with (6) additional unknown intermediate factors, increase myocyte hypertrophy, contractile function, and cardioprotection (Karpanen et al., 2008b; Zentilin et al., 2010; Kivelä et al., 2014; Lal et al., 2017). Bottom panel: (1) Axitinib, a pan-tyrosine kinase inhibitor, prevents downstream VEGF-VEGFR signaling. (2) VEGF-induced inhibition of EPO is interrupted, resulting in an increase in EPO-E and EPO-C production. The net outcome of VEGF-VEGFR inhibition is impaired cardiac function and bradycardia, despite increased EPO production. The mechanisms underlying these physiological consequences require future work.