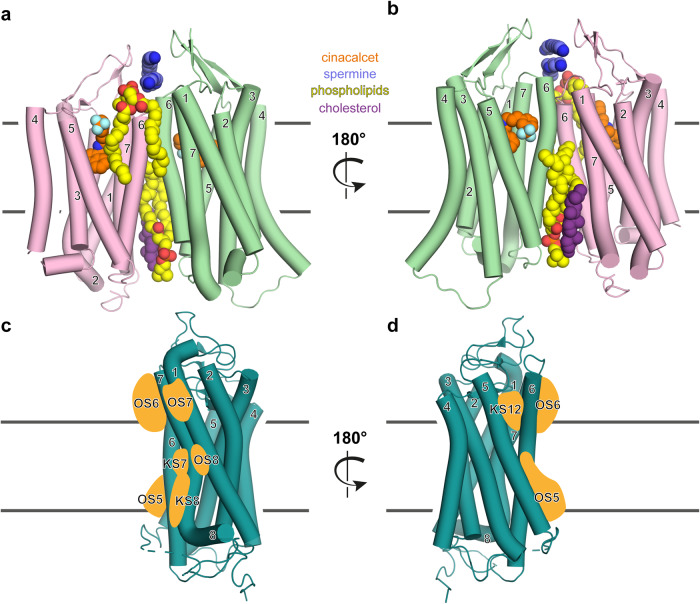
Fig. 1.
Exploring allosteric CaSR ligand binding and the GPCR pocketome. a, b Cryo-EM structure of the CaSR dimer with numbered TM helices, similarly color-coded as in the original study.1 The active protomer, interacting with the G protein (not shown) is illustrated in green and the inactive protomer in pink. The large extracellular ligand binding domain is not shown to enable a detailed depiction of the classical 7TM helical domain, similar to d, e in the original publication.1 In the structure many different interacting molecules were resolved: The calcimimetic drug cinacalcet (orange, blue), located in a comparable orthosteric binding pocket of other GPCR families, spermine (dark blue), above the TM dimer interface, phospholipids (yellow, red) at the front of the dimer (a), as well as the back of the dimer (b), in addition to cholesterol (purple). PDB: 8SZG, 8SZH. X-ray structure of a class A GPCR with numbered TM helices from front (c) and back (d), similarly oriented as the active protomer (green) above (a, b). Based on the analysis of more than 500 class A GPCRs and their pocketome of allosteric ligand binding sites by ref. 5 potentially analogous utilized binding sites in the class C CaSR structure are schematically represented as orange shapes (orphan site (OS) 5 (middle and lower portion (MP, LP, respectively) TMV, TMVI), OS6 (upper portion (UP) TMVI, TMVII), OS7 (UP TMI, TMVII), OS8 (MP TMI, TMVII); known sites (KS) 7 (MP TMVI, TMVII), KS8 (LP TMVI, TMVII), KS12(UP TMV, TMVI))