FIGURE 2.
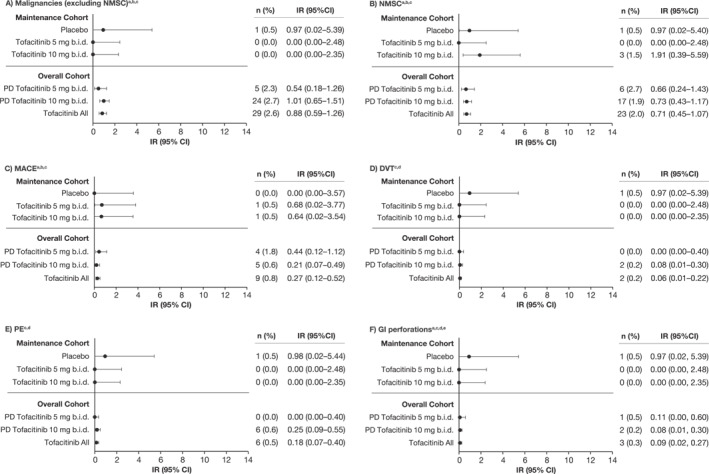
IRs (unique patients with events per 100 PY of exposure) for (a) malignancies (excluding NMSC), (b) NMSC, (c) MACE, (d) DVT, (e) PE, and (f) GI perforations in the tofacitinib UC clinical program, by cohort. Data for the Maintenance Cohort have been reported previously. 5 , 6 Briefly, the Maintenance Cohort comprised patients who received placebo or tofacitinib 5 or 10 mg b.i.d. for 52 weeks in OCTAVE Sustain 5 , 6 ; and the Overall Cohort comprised patients who received ≥1 dose of tofacitinib 5 or 10 mg b.i.d. in any phase 2/3/OLE study plus final data from the phase 3b/4 data as of 18 April 2022 (≤9.2 years of exposure). Exact Poisson distribution was used to calculate IR 95% CI. b.i.d., twice daily; CI, confidence interval; DVT, deep vein thrombosis; GI, gastrointestinal; IR, incidence rate (unique patients with events per 100 PY of exposure); MACE, major adverse cardiovascular events; N, number of patients treated in the treatment group; n, number of unique patients with a particular adverse event; NMSC, non‐melanoma skin cancer; OLE, open‐label, long‐term extension; PD, predominant dose; PE, pulmonary embolism; PY, patient‐years; UC, ulcerative colitis.a for the Overall Cohort, N = 905 and N = 1124 for the PD tofacitinib 10 mg b.i.d. and tofacitinib all groups, respectively (excludes phase 2). bFor the Maintenance Cohort, events that occurred >28 days after the last dose of the study drug were excluded; for the Overall Cohort, events outside the 28‐day risk period were included. cAdjudicated events. dEvents that occurred >28 days after the last dose of the study drug were excluded. eGI perforation excludes Preferred Terms of pilonidal cyst, perirectal abscess, rectal abscess, anal abscess, perineal abscess and any Preferred Terms containing the term fistula.
