Abstract
The use of acid suppression therapy (AST) is a common approach for managing a wide spectrum of acid peptic disorders. Histamine type 2-receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) are the most widely prescribed AST in routine clinical practice. However, an exponential surge in the prescriptions of PPIs, such as Omeprazole, Esomeprazole, Pantoprazole, Lansoprazole in recent years and their associated adverse effects have raised concern about their inappropriate and overuse, both in children and adults. To address these issues, a three-step modified Delphi polling process was employed to establish best practice consensus statements for rationalizing the use of acid suppressants. A multidisciplinary expert panel of 13 health professionals across medical specialties, including gastroenterologists, hepatologists, pediatric gastroenterologists, pediatricians, otolaryngologists, cardiologists, nephrologists, gynecologist and orthopedists actively contributed to this collaborative process of consensus development. The expert panel proposed 21 consensus statements providing best practice points on the general use and safety of acid suppressants based on a comprehensive review of scientific literature and clinical expertise. The panel also collaboratively developed a PPI deprescribing algorithm. Altogether, this consensus paper offers evidence-based recommendations and guidance for the rational use of acid suppressants with a blueprint for deprescribing PPIs. This consensus paper contributes to aiding primary care practitioners in improving patient outcomes and minimizing healthcare costs. Additionally, it enhances patient safety and curtail inappropriate usage.
How to cite this article
Prabhoo RY, Pai UA, Wadhwa A, et al. Multidisciplinary Consensus for Rationalizing the Use of Acid Suppressants in Children and Adults: CONFOR. Euroasian J Hepato-Gastroenterol 2024;14(1):99–119.
Keywords: Deprescribing, Gastrointestinal, H2RAs, Proton pump inhibitors, Ranitidine, Side effects
Introduction
Acid suppression therapy (AST) is the mainstay for effectively managing acid peptic disorders.1 Histamine type 2-receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) are the most commonly prescribed ASTs, which work through two different mechanisms to suppress gastric acid secretion. H2RAs inhibit gastric acid secretion through competitive inhibition of histamine H2 receptors of the gastric parietal cells, while PPIs inhibit H+/K+-ATPase (proton pump) in the gastric parietal cells.2 Potassium competitive acid blockers (P-CABs) represent a recent addition in the realm of AST. Clinical trials have demonstrated promising results for P-CABs across a range of indications, with some studies showing superior efficacy over PPIs in indications such as Helicobacter pylori (H. pylori) eradication and erosive esophagitis (EE). While, in other gastric acid-related diseases, P-CABs have demonstrated non-inferiority to existing treatments.3 However, P-CABs are not yet available for use in India.
Over the last two decades, PPIs have been preferred over H2RAs due to their potent acid-suppressive effect.4 However, the exponential growth in PPI prescriptions has increased the concern about its overutilization and misuse. Even though the duration of treatment for the majority of the indications is for short-term, over-the-counter availability, the perception among both patients and healthcare practitioners that these medications are harmless has led to their inappropriate use. In many patients, these drugs are prescribed without a clear indication or continued for an extended duration without reassessment. Multiple studies have documented that in nearly half of the cases, PPIs are prescribed inappropriately. In an observational study, approximately 56% of the indications to initiate PPI therapy were inappropriate in primary care settings.5 In another retrospective study, prescription appropriateness analysis showed that 47% of the new PPI users were prescribed PPIs for unapproved indications.6 A study from Southeast Asia reported that 81% of the studied patients had no documented indications for PPI use.7 Multiple studies form western countries have also provided compelling evidence of inappropriate utilization of PPIs.7 The implications of inappropriate and overuse of PPIs not only expose patients to potential risks but also increase overall healthcare costs.
Recent literature has raised several concerns about the safety profile of acid suppressants, particularly PPIs, when used for the long term unless clinically indicated. This highlights the need for caution with appropriate prescription and use of AST across the age groups.2
In light of these compelling factors, the development of a multidisciplinary consensus paper with a comprehensive review to rationalize the use of acid suppressants, specifically PPIs, becomes imperative. Hence, a panel of experts representing diverse medical specialties, including gastroenterologists, hepatologists, pediatric gastroenterologists, pediatricians, cardiologists, nephrologists, an otolaryngologist, a gynecologist, and an orthopedist, collaborated to develop best practice consensus statements.
The primary objective of this consensus paper was to provide evidence-based practice points for rationalizing the use of acid suppressants, with a primary focus on PPIs. These consensus statements intend to provide general practitioners and other primary care physicians with recommendations for appropriate prescription of AST in their routine clinical practice. Also, this paper presents a simplified PPI deprescribing algorithm, which would serve as a practical tool to assess the appropriateness of ongoing therapy and provide guidance on when and how to taper the dose or stop its use.
Materials and Methods
The three-step modified Delphi polling process was used to reach a CONsensus among the multidisciplinary panel of healthcare professionals FOR rationalizing and deprescribing acid suppressants in children and adults (CONFOR). The process of assembly of the panel was initiated in April 2023. The panelists were carefully selected by the national coordinator based on their active involvement in clinical practice and research within their respective specialties. A total of 15 experts were invited to collaborate in the development of this consensus paper, with acceptance received from 13 of these experts. The panel consisted of two gastroenterologists, two pediatric gastroenterologists, two pediatricians, a hepatologist, two cardiologists, a nephrologist, an otolaryngologist, a gynecologist, and an orthopedist. This multidisciplinary panel was chaired, and the consensus development process was moderated by the national coordinator.
A virtual meeting was held among the participating experts on 15th June 2023 to introduce the panelists and to discuss the literature search criterion, the scope of the recommendations, and the process of developing a consensus document. Following the virtual meeting, an extensive literature search was done on decided topics for relevant articles, which were published before July 2023 on PubMed and Google Scholar. In addition, a manual search of the bibliographies of potential articles was also carried out using the Google search engine. The literature review included clinical trials, cohort studies, systemic reviews and meta-analyses, expert consensus papers, and professional society guidelines. The dossier of the compiled literature was sent to all the participating experts. The national coordinator of the panel, in collaboration with other experts, formulated initial consensus statements through a comprehensive review of scientific literature. The proposed statements were directed toward four categories, including general statements pertaining to the appropriate use of AST, safety, monitoring, and deprescribing AST with specific emphasis on PPIs.
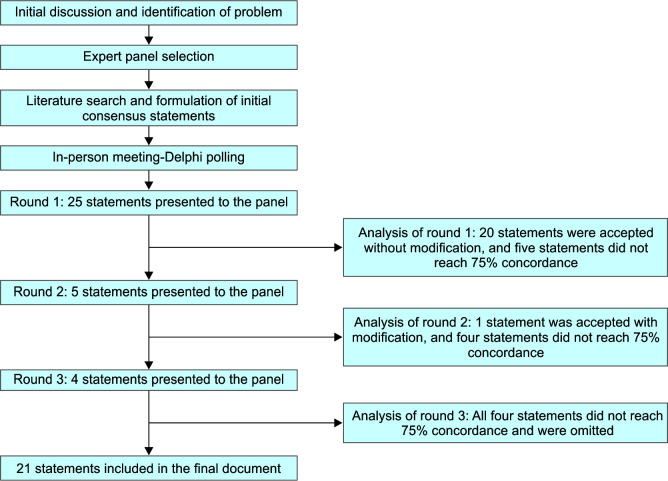
The first physical meeting of the multidisciplinary expert consensus group was conducted on 13th August 2023 in Mumbai, Maharashtra, India. For each proposed consensus statement, all members of the multidisciplinary group voted on a 5-point Likert scale (ranging from “strongly agree” to “strongly disagree”) using an electronic voting platform. During the Delphi polling, statements for which at least 75% of the experts collectively voted “strongly agree” or “agree” were considered to have achieved consensus and were accepted to be included in the consensus paper without further deliberation. Figure 1 depicts the process of consensus development and steps in the modified Delphi method. During the first round, 25 statements were presented for polling, of which 20 statements were accepted without modification, and five statements did not reach 75% concordance. These five statements were modified based on the experts' suggestions. Any disagreements among the panelists were resolved with discussion. Subsequently, polling was done on these five modified statements during the second round of Delphi polling, where one statement was accepted, and four statements did not reach 75% concordance. During the third and final round, four statements were discussed for further modification; however, all these statements did not reach 75% concordance and were omitted from the final document. The accepted 21 statements were finally adopted as consensus recommendations. Based on the evidence and clinical considerations, the panel of experts assigned strength to each recommendation. The gradations included strong, conditional, and weak recommendations. Strong recommendation is considered when the evidence is robust and the benefits clearly outweigh the risks. A conditional recommendation is considered when the evidence is less certain, and there may be a balance between benefits and risks, whereas a weak recommendation is considered when the evidence is inadequate, and the balance between the benefit and risk is uncertain.
Fig. 1.
Process of consensus development and steps in the modified Delphi method
This multidisciplinary group also collaboratively developed a PPI deprescribing algorithm based on the published literature and their clinical experience. The initial algorithm was reviewed and modified until a consensus was reached among all the experts.
Each panel member equally played a significant role in the discussion, demonstrating active engagement throughout the consensus development process. The experts were independent in their decision-making and were not subject to any external influences or conflicts of interest. The manuscript was drafted by the writing committee and was reviewed by all the members of the expert panel, ensuring a comprehensive and well-structured draft.
Appropriate and Inappropriate Use of ASTs
Appropriate Use of ASTs
There are a wide range of approved indications of AST, including the treatment of gastroesophageal reflux disease (GERD), EE, dyspepsia, peptic ulcer disease (PUD), hypersecretory disorders, such as Zollinger-Ellison syndrome, Helicobacter pylori eradication, and stress ulcer prophylaxis (SUP) in high-risk patients.8 PPIs are the most commonly prescribed AST indicated for managing upper GI conditions such as peptic ulcers and GERD. They are favored for their effective control of both basal and meal-stimulated acid secretion, leading to prolonged increases in gastric pH values. Unlike H2RAs, PPIs exhibit a relatively slow onset of action, making them less suitable for on-demand use.4,8,9
Short-term PPI therapy is generally adequate for managing most acid peptic disorders, for which the therapy typically lasts for 4–8 weeks. On the contrary, prolonged PPI therapy should be reserved for specific indications, such as prophylactic use in chronic NSAID users, Zollinger-Ellison syndrome, severe EE, Barrett's esophagus, and eosinophilic esophagitis. In typical cases of GERD, PPIs are effective as a short-term treatment (4–8 weeks) for both EE and non-erosive reflux disease (NERD).4,8–11
It is well documented that not all patients receiving NSAIDs require PPI prophylaxis. For the prevention of NSAID-associated gastric ulcers, it is essential to identify and address various risk factors that may necessitate the use of PPIs.12 According to the American Gastroenterology Association (AGA) guidelines, these risk factors include prior history of gastric ulcer, elderly patients aged 65 years and above, concurrent use of NSAIDs (like aspirin, ibuprofen, and diclofenac) in combination with other gastrotoxic drugs which can amplify the risk of gastric injury. Identifying and addressing these risk factors is crucial in mitigating the potential for drug-induced gastric ulcers and associated complications. However, whether prophylactic use with PPIs should be continued to prevent the development of ulcers is still debatable.12 On the contrary, AGA advises the use of PPIs for short-term healing and long-term symptom control for patients with GERD and acid-related complications such as EE or peptic stricture. The guideline also recommends that attempts should be made to reduce or discontinue PPIs for patients with uncomplicated GERD. Periodic assessment is recommended to prescribe the lowest effective PPI dose for the shortest duration. Long-term PPI therapy is advised for symptomatic GERD in Barrett's esophagus patients, but for asymptomatic Barrett's patients, the decision should be personalized due to limited evidence.12
For patients receiving dual-antiplatelet therapy (DAPT), prophylactic use of PPI is recommended only in patients with a history of previous GI bleeding, patients with increased risk of GI bleeding, and those with concomitant use of NSAIDs, warfarin, or steroids.12–14 Recently, there has also been an increase in the prescription of PPIs in pediatric patients. These prescriptions are primarily for the treatment of conditions, such as eosinophilic esophagitis, reflux symptoms, peptic ulcer disease, and H. pylori eradication. Notably, the efficacy of PPI for these indications in pediatric patients appears to be comparable to that observed in the adult population, making them the most appropriate indications in pediatric patients.15,16
Table 1 summarizes the appropriate indications of PPIs in adults and children based on a thorough literature search and the opinions of experts who participated in the consensus development.
Table 1.
Appropriate use of PPI in adults and children
| Appropriate indications of PPI in adults | Appropriate indications of PPI in children |
|---|---|
| Healing of EE and maintenance to prevent relapse | Pathologic reflux symptoms in children >1 year |
| PPI-responsive eosinophilic esophagitis | Peptic ulcer disease |
| Peptic ulcer disease | Eosinophilic esophagitis |
| GERD | H. pylori eradication |
| Relief of symptoms in patients with NERD | |
| Barrett's esophagus | |
| Zollinger-Ellison syndrome | |
| Short-term treatment of functional dyspepsia | |
| Eradication of H. pylori infection | |
| Prophylaxis and healing of NSAID-associated gastric ulcers, and prophylaxis of gastrointestinal side effects of antiplatelet or anticoagulant therapy in high-risk patients. | |
| SUP in critically ill patients at risk |
GERD, gastroesophageal reflux disease; NERD, non-erosive reflux disease; PPIs, proton pump inhibitors; SUP, stress ulcers prophylaxis
Inappropriate Use of ASTs
Inappropriate use of AST is an increasing concern in current healthcare settings, leading to overuse of medications, increased cost of therapy, and potential adverse effects. In a study evaluating the appropriateness of AST, approximately 43% of patients who received AST did not have appropriate indications for its use.17 Among ASTs, the use of PPIs continues to grow year by year despite the fact that their clinical indications have remained unchanged for many years. Multiple studies have reported that the rate of inappropriate use of AST, especially PPIs, is around 50% among patients under the care of general practitioners and approximately 57% among hospitalized patients. This inappropriate use of PPIs is alarming since these medications are prescribed for off-label use in more than half of cases. In addition to unapproved indications, unwarranted and prolonged treatment is also accountable for the inappropriate use of PPIs (Table 2).4
Table 2.
Inappropriate use of PPIs in children and adults
| Inappropriate indications of PPI |
|---|
| Routine use in patients with mild, infrequent heartburn |
| Non-specific upper abdominal discomfort without a confirmed diagnosis |
| Long-term use without reevaluation |
| Routine prophylactic use with primary care treatment of non-ulcerogenic nature |
| Routine prophylactic use with NSAIDs and antibiotics |
| Routine prophylactic use with antiplatelet therapy in low-risk patients |
| SUP in low-risk, non-ICU hospitalized patients |
| Management of extra-esophageal symptoms |
ICU, intensive care unit; NSAIDs, non-steroidal anti-inflammatory drugs; SUP, stress ulcer prophylaxis
Hospitalized patients are frequently prescribed PPIs along with primary care treatment, even if it is not clinically indicated.4 Routine prophylactic use of PPIs with antiplatelet therapy in low-risk patients also seems to be inappropriate.12–14 The use of AST for SUP in an ICU setting is well documented. However, this practice has been extended to low-risk non-ICU hospitalized patients as well. Previous reports have indicated that inappropriate AST prescription rates in non-ICU hospital settings range from 40 to 76%. These findings highlight the need for a critical reassessment of AST use in non-ICU settings.18 Patients discharged from hospitals also often continue AST, particularly PPIs, without appropriate indications.4 In primary care settings, misdiagnosis of acid-related disorders with functional heartburn could also lead to unwarranted and prolonged use of AST inappropriately. Patients' concerns about gastric issues associated with antibiotics, chemotherapeutic agents, and bisphosphonates, when administered alone, could also lead to the overuse of potent AST, like PPIs.4 On the other hand, PPIs have shown limited evidence for the treatment of gastroesophageal reflux in neonates and infants. It has been widely advised that PPI therapy in this age group should be reserved only in cases with clear evidence of pathological exposure to acid and/or in patients with esophagitis.19,20 In children, ASTs are also inappropriately prescribed for the management of extra-esophageal symptoms like cough associated with GERD.16 Despite the explicit recommendations, there has been a significant increase in the prescription of PPIs in infants and toddlers. This increasing trend has raised growing safety concerns regarding the use of ASTs across the age group.19,20
AST-associated Side Effects and Complications
Side Effects Associated with the Use of AST in Adults
The emerging evidence on side effects associated with AST, specifically with chronic use of PPIs, raises concern about the irrational use of PPIs that prompts the use of safer alternatives like H2RAs (e.g., ranitidine, famotidine, nizatidine) in clinical practice. Here, we have reviewed the most studied and well documented side effects associated with ASTs. Table 3 summarizes the most reported side effects associated with AST, particularly PPIs.
Table 3.
Summary of well documented side effects associated with ASTs (especially PPIs) in adults and children
| Side effects reported in adults | Side effects reported in children |
|---|---|
| Nutritional deficiencies | Alteration of microbiome |
| PPI-induced hypomagnesemia | Clostridium difficile infections (CDI) |
| Iron deficiency anemia | Serious infections |
| Vitamin B12 deficiency | Asthma |
| Gastroenterological side effects | Fractures |
| Dysbiosis | Hospital-acquired acute kidney injury (AKI) |
| Clostridium difficile infections (CDI) | |
| Inflammatory bowel disease (IBD) | |
| Bacterial peritonitis | |
| Fundic gland polyps | |
| Microscopic colitis | |
| Renal side effects | |
| Acute interstitial nephritis (AIN) | |
| Acute kidney injury (AKI) | |
| Chronic kidney disease (CKD) | |
| End-stage renal disease (ESRD) | |
| Cardiovascular complications | |
| Cardiovascular disease | |
| Ischemic stroke | |
| Heart failure | |
| Myocardial infarction | |
| Side effects related to bone and joints | |
| Increased propensity of fractures | |
| Functional decline | |
| Prosthetic joint infection | |
| Bone loss |
Nutritional Deficiencies Associated with AST Use
Chronic acid suppression, mainly with PPIs, is associated with impaired absorption of calcium, iron, and magnesium, increasing concerns regarding bone health, and heightened fracture risk.21 The use of PPIs has also been linked to an increased likelihood of vitamin and mineral deficiency. These deficiencies include vitamin B12 and C, especially in elderly, malnourished individuals, and chronic hemodialysis patients.22 Table 4 summarizes key studies linking PPI use and nutritional deficiencies.
Table 4.
Key studies highlighting nutritional deficiencies associated with AST
| Study | Study characteristic | Key findings |
|---|---|---|
| Hypomagnesemia | ||
| Kieboom et al.23 | Population-based prospective cohort study N = 9,818 individuals from the general population | Prolonged PPI use was associated with lower levels of serum magnesium level and significantly increased risk of PPIH (OR, 2.00) Concomitant loop diuretic use further increases the risk The risk was less in H2RA users |
| Cheungpasitporn et al.24 | Meta-analysis of nine observational studies N = 1,09,798 patients | PPI use was associated with a 43% increased risk of hypomagnesemia |
| Srinutta et al.25 | Meta-analysis of 16 observational studies N = 1,31,507 patients | PPI use was significantly associated with hypomagnesemia (OR, of 1.83; p = 0.002) High-dose PPI use was associated with higher odds relative to low-dose PPI use (OR 2.13; p = 0.005) |
| Seah et al. 202326 | Retrospective study N = 53,149 patients | Higher recurrence of hypomagnesemia in patients continuing PPI therapy (p = 0.009) |
| Iron deficiency | ||
| Sarzynski et al.27 | Retrospective cohort study N = 98 patients on chronic PPI therapy | Chronic PPI therapy resulted in a reduction in hematologic indices compared with baseline, including a decrease in hemoglobin (–0.19 gm/dL, p = 0.03), hematocrit (–0.63%, p = 0.02), and mean corpuscular volume (–0.49 fL, p = 0.05). |
| Tran-Duy et al.28 | Case-control study N = 26,806 patients with iron deficiency | Chronic PPI use increases the risk of iron deficiency (OR, 3.60) A clear association was seen between iron deficiency and PPI use in terms of both duration of treatment and PPI dosage. Continuous use of PPIs for one year or more is linked to elevated iron deficiency risk. Average daily dosage of PPIs equal to or exceeding 1 DDD have a higher risk of iron deficiency. |
| Douwes et al.29 | Cross-sectional cohort study N = 646 stable outpatient RTR | PPI use was inversely associated with serum iron (p = 0.001), natural log-transformed serum ferritin (p < 0.001), TSAT (p = 0.001), and hemoglobin levels (p = 0.007)
A higher risk of iron deficiency was observed in patients taking high dosages of PPIs |
| Vitamin B12 deficiency | ||
| Dharmarajan et al.30 | Cross-sectional study N = 659 adults >60 years age | Vitamin B12 levels decline during prolonged PPI use in older adults; however, prolonged H2RA use did not affect vitamin B12 status |
| Mumtaz et al.31 | Cohort study N = 1,225 patients on long-term use of PPIs | Patients taking PPIs were more likely to develop vitamin B12 deficiency. More than half of the men exhibited low levels of vitamin B12 |
DDD, defined daily dose; H2RA, histamine-2 receptor antagonist; ICU, intensive care unit; OR, odds ratio; PPI, proton pump inhibitor; PPIH, proton pump inhibitor-induced hypomagnesemia; RTR, renal transplant recipients; TSAT, transferrin saturation
Hypomagnesemia
The concern of PPI-induced hypomagnesemia (PPIH) first emerged in 2006 and has since been substantiated by numerous reports and studies.32 The existing evidence highlights a complex interplay of factors, including molecular biology, pharmacology, and genetic predisposition.33 The literature also clearly indicates that PPIH is frequently accompanied by hypocalcemia, hypoparathyroidism, and hypokalemia.32 Key studies reporting the association between PPI use and hypomagnesemia are presented in Table 4. Observational studies have consistently shown that PPI users have an increased risk of hypomagnesemia, especially with prolonged use and concurrent loop diuretic use. Meta-analyses further support this association, indicating a dose–response relationship with high-dose PPI users at greater risk.24,25 Several risk factors contributing to the development of hypomagnesemia have been identified, including factors such as female gender, diabetes mellitus, low body mass index (BMI), high-dose PPI usage, renal dysfunction, and the use of diuretic medications.26 The prevalence of PPIH is notably higher in older patients, especially males, smokers, and those with alcohol consumption exceeding seven units per week.34 Discontinuing PPIs can lead to rapid recovery, but reintroduction or switching to another PPI can result in recurrence.26 In contrast, H2RAs like ranitidine do not appear to cause this complication, suggesting H2RAs as a potential alternative for patients with hypomagnesemia.23,35,36 Overall, the evidence highlights the importance of recognizing and managing PPI-induced hypomagnesemia to ensure patient safety, particularly in susceptible individuals.
Iron Deficiency
The collective findings from multiple studies on PPI use reveal a positive correlation with iron deficiency (Table 4). Long-term use of PPIs, even in cases where there is no clear indication for use, is linked to a significant reduction in hematologic indices, particularly hemoglobin and hematocrit levels. This suggests a potential association between chronic PPI use and iron deficiency anemia.27 A dose–response and time–response relationship has been established between chronic PPI use and the risk of iron deficiency in a large cohort, emphasizing the importance of considering the duration and dosage of PPIs when assessing the risks and benefits of long-term prescriptions.28 Among stable renal transplant recipients, chronic PPI use is independently associated with lower iron status and an increased risk of iron deficiency. These findings suggest the need for monitoring and adjusting PPI dosage in this patient population.29 Intravenous iron replacement therapy has been shown to effectively correct iron deficiency in patients taking PPIs who were non-responsive to oral iron therapy.37 In clinical practice, these findings suggest the need for judiciously prescribing PPIs or considering alternative approaches to mitigate the risk of iron deficiency and its associated complications.
Vitamin B12 Deficiency
Prolonged and high-dose PPIs have long been associated with vitamin B12 deficiency, with varying prevalence rates reported in the literature. Table 4 enlists key studies on PPI usage associated with vitamin B12 deficiency.
Clinical manifestations of vitamin B12 deficiency range from mild anemia to severe neurodegenerative impairments. Elevated homocysteine levels, indicative of vitamin B12 deficiency, are linked to potentially increased cardiovascular disease risk.38 Additionally, studies have reported that long-term PPI users are at an increased risk of vitamin B12 deficiency.31 A study involving older adults revealed that PPI use, but not H2RAs, led to a significant decline in serum B12 levels, even when oral vitamin B12 therapy. This highlights the importance of monitoring vitamin B12 levels during prolonged PPI therapy to prevent serious complications, especially cardiovascular and neurodegenerative impairments.30
Gastrointestinal Side Effects Associated with AST
Now, it is well documented through evolving evidence that prolonged use of AST, especially PPIs, is associated with an increased risk of a wide range of gastrointestinal side effects compared with H2RAs such as ranitidine, famotidine, etc. Table 5 summarizes key studies associated with various gastrointestinal side effects associated with PPIs.
Table 5.
Studies reporting gastrointestinal side effects associated with AST
| Study | Study characteristic | Key findings |
|---|---|---|
| Dysbiosis | ||
| Jackson et al.39 | Population-based cohort analysis N = 1,827 individuals from the TwinsUK study | Significantly lower abundance in gut commensals and lower microbial diversity were reported in PPI users |
| Hojo et al.40 | Observational study N = 20 patients with reflux esophagitis who received 8-week PPI therapy | A significant increase in Lactobacillus species and Streptococcus species in fecal samples was observed following 4 and 8 weeks of treatment with PPIs, respectively |
| Horvath et al.41 | Cohort study N = 50 patients with cirrhosis on long-term PPI therapy and 40 control patients with cirrhosis without PPI therapy | Patients on long-term PPI therapy showed a significant increase in Streptococcus salivarius, Veillonella parvula, and the genus Streptococcus, which performed well as biomarkers for dysbiosis |
| Clostridium difficile infection | ||
| Barletta and Sclar, 201442 | Retrospective case-control study N = 400 ICU patients | PPI use was an independent risk factor for development of CDI in ICU patients (p = 0.012) The heightened risk was particularly associated with two or more days of PPI therapy |
| Trifan et al.43 | Systematic review and meta-analysis of 56 studies N = 3,56,683 patients | PPI use increased the risk of CDI (OR, 1.99; p < 0.001) |
| Tariq et al.44 | Systematic review and meta-analysis N = 7,703 patients with CDI | A higher rate of recurrent CDI (22.1%) was observed in patients receiving AST |
| Park et al.45 | Single-center, cohort study N = 3,09,073 hospitalized patients, n = 1,25,922 PPI users, n = 1,83,151 | PPI use was associated with a 1.8-fold increase in CDI risk High-dose PPI increased the risk of CDI by two-fold |
| Seo et al.46 | Retrospective, observational, comparative cohort study N = 67,915 patients with CDI | There was a significantly higher risk of CDIs among patients taking PPIs compared with those on H2RAs HR for CDI development was 2.22 times higher in the PPI group compared with the group of patients receiving H2RAs |
| Inghammar et al.47 | Nationwide cohort study N = 3,583 cases of community-associated CDI | PPI users showed a 2.03-fold increased risk of community-associated CDI compared with nonusers |
| D'Silva et al.48 | Systematic review and meta-analysis N = 57,477 patients with CDI | Significantly higher odds of recurrent CDI were observed in patients who received PPIs (OR, 1.69) |
| Lee et al.49 | Retrospective cohort study N = 16,820 ICU patients | A higher proportion of patients receiving PPI developed CDI compared with H2RAs (3.0 vs 0.8%, p < 0.001) Both intravenous (OR, 2.4) and oral (OR, 2.3) use of PPI were associated with higher odds of developing CDI compared with H2RAs |
| Inflammatory bowel disease | ||
| Xia et al.50 | Pooled analysis NHS, n = 82,869; NHS II, n = 5,141; and UK Biobank, n = 4,69,397 |
PPI use was consistently associated with a significantly increased risk of IBD A high risk of IBD was also observed in regular PPI users compared with H2RAs alone |
| Shastri et al.51 | Systematic review and meta-analysis of eight studies N = 1,57,758 participants | PPI use increased the risk of IBD (adjusted OR, 2.43), including ulcerative colitis and Crohn's disease together, collagenous colitis, and lymphocytic colitis |
| Onwuzo et al.52 | Cross-sectional population-based analysis N = 45,586,150 | PPI use increased the odds of ulcerative colitis (OR, 2.02; p < 0.001) as well as Crohn's disease (OR, 2.79; p < 0.001) development |
| Choden et al.53 | Longitudinal, retrospective cohort analysis N = 46,234 patients with IBD, n = 6,488 PPI users, n = 39,746 nonusers | PPI use in IBD patients was associated with higher odds of hospital admissions (OR, 1.95) and surgeries (OR, 1.46) Worse clinical outcomes were observed in IBD patients on concomitant PPI therapy |
| Bacterial peritonitis | ||
| Min et al.54 | Retrospective cohort study N = 1,554 cirrhotic patients with ascites | PPI group patients had a higher annual SBP incidence rate compared with nonusers PPI use was an independent risk factor for SBP (HR, 1.396) |
| Xu et al.55 | Comprehensive meta-analysis N = 8,204 cirrhotic patients with ascites | PPI use was significantly associated with an increased risk of SBP (OR, 2.17) and overall bacterial infection (OR, 1.98) in cirrhotic patients with ascites |
| Zang et al.56 | Retrospective study N = 1,092 patients with chronic liver diseases/cirrhosis with acute insults (the HBV-reactivation) | PPI users with elevated MELD scores were at an increased risk of SBP |
| Dahabra et al.57 | Retrospective cohort analysis N = 1,07,750 patients with SBP | PPI use was strongly associated with an increased risk of SBP (OR, 4.24; p < 0.0001) compared with nonusers |
| Fundic gland polyps | ||
| Ally et al.58 | Retrospective cohort study N = 385 patients who underwent upper endoscopy | Chronic PPI therapy lasting for more than 48 months was an independent predictor of FGP (OR, 4.7; p = 0.001) |
| Tran-Duy et al.59 | Systematic review with a meta-analysis of 12 studies N = 87,000 patients with FGP | Long-term PPI use (≥12 months) was associated with a significantly increased risk of FGP (OR, 1.43) |
| Martin et al.60 | Systematic review and meta-analysis of 12 studies N = 40,218 patients with FGP | PPI usage increased the odds of FGP development (OR, 2.46; p = 0.001) This association was more pronounced in individuals on PPI therapy for at least six months (OR, 4.71) or 12 months (OR, 5.32) |
| Velazquez-Dohorn et al.61 | Case-control analysis N = 133 patients with gastric polyps | PPI administration for at least one year was linked to the development of gastric FGP (OR, 7.7) |
| Microscopic colitis | ||
| Keszthelyi et al.62 | Retrospective case-control study N = 136 cases of microscopic colitis | Histological diagnosis of microscopic colitis was significantly associated with exposure to PPIs compared with controls (38 vs 13%; p < 0.001; adjusted OR, 4.5) |
| Masclee et al.63 | Population-based nested case-control study N = 1,458,410 subjects | The use of PPIs (OR-adjusted 10.6) and NSAIDs (OR-adjusted 5.6) increased the risk of microscopic colitis |
| Bonderup et al.64 | Nationwide case‐control study N = 10,652 patients with a diagnosis of microscopic colitis | There was a robust association between current PPI use and both collagenous colitis (adjusted OR, 6.98) and lymphocytic colitis (adjusted OR, 3.95) |
AST, acid suppression therapy; CDI, Clostridium difficile infections; FGP, fundic gland polyps; H2RAs, histamine-2-receptor antagonists; HBV, hepatitis B virus; HR, hazard ratio; IBD, inflammatory bowel disease; NHS, Nurses' Health Study; MELD, model for end-stage liver disease; OR, odd ratio; SBP, spontaneous bacterial peritonitis
Dysbiosis
It has been proven in multiple studies that over use of acid suppressants, especially PPIs, has been associated with significant alterations in the gut microbiota, raising concerns about potential health hazards (Table 5).65 Several studies suggest that PPIs adversely affect the normal gut microbiota, leading to dysbiosis. Such changes may weaken the ability of the gut to resist infections, exacerbate inflammation, and eventually predispose an individual to gastrointestinal disorders.39,66 Long-term PPI use may cause achlorhydria in some cases, which may promote the migration of oral bacteria into the lower gut, increasing the chances of infections.40,66 In cirrhotic patients, PPI therapy has been linked to disruptions in the gut microbiome, spontaneous bacterial peritonitis, and hepatic encephalopathy. Identification of specific species, such as Streptococcus salivarius, Veillonella parvula, and the Streptococcus genus, were indicative of PPI-associated dysbiosis, intestinal inflammation, and severe liver disease.41 Overall, these findings highlight the impact of PPIs on gut microbiota and its implications for increasing the risk of infection.
Clostridium difficile Infection
Clostridium difficile infection (CDI) is prevalent in both hospitalized and community-dwelling patients. Approximately, one in every five patients with initial CDI experience recurrence. Hence, it is imperative to identify modifiable factors that can reduce the risk of both initial and recurrent CDI.67 Studies evaluating the association between the use of AST and CDI infection have demonstrated a 50% higher likelihood of recurrence in patients using acid suppressants when compared with those who do not use these medications (Table 5).67 The comprehensive analysis of multiple studies highlights a significant association between the use of PPIs and the risk of CDI. Studies consistently demonstrate that PPI use increases the risk of both initial and recurrent CDI.68
In community settings, PPI use is linked to a 1.8–2.0-fold increase in the risk of CDI, with greater risk at higher doses. Short-term exposure to PPIs, for <1 week, has also been reported to increase the risk of CDI by 4.2-fold in some cases.45 A nationwide cohort study revealed that PPI users were associated with a 2.03-fold increased risk of community-associated CDI compared with nonusers.47 A strong association between PPI therapy and CDI risk has been confirmed in a systematic review and meta-analysis involving a large number of patients with a significant increase in CDI risk (p < 0.001) in PPI users.43 Also, the risk of hospital-acquired CDI is increased by 38.6% with PPI use. Moreover, ICU patients receiving PPIs, either intravenous or oral, are also at an increased risk of CDI. Long-term PPI use, particularly for two or more days, is an independent predictor of CDI development in ICU patients. These findings suggest the need for careful consideration of PPIs in hospital settings, especially in intensive care units.42,67 In both community and hospital settings, PPIs are associated with a higher risk of CDI when compared with H2RAs.46,49,69 The association between AST and recurrent CDI remains consistent, with a higher recurrence rate in PPI users, as demonstrated in multiple observational studies and meta-analyses.44,48,70
Inflammatory Bowel Disease
Several studies have investigated the clinical outcomes and risk of IBD associated with the use of PPIs (Table 5). A large population study involving adult IBD patients found that those using PPIs were more likely to initiate new biological treatments and increased hospitalization. In addition, a dose–response relationship indicated that high-dose PPI use was correlated with adverse outcomes.53
Another study reported a higher rate of PPI use among individuals with IBD, particularly in the first year following diagnosis, suggesting a potential link between PPI use and the development of IBD.71 A pooled analysis of different cohorts demonstrated a consistent association between regular PPI use and an increased risk of IBD as compared with H2RAs.50 A systematic review and meta-analysis of multiple studies also confirmed a significant association between PPI use and a higher risk of IBD.51 A cross-sectional population-based analysis using a vast database of over 45 million patients revealed a significant association between PPI use and the development of both ulcerative colitis (OR, 2.02; p < 0.001) and Crohn's disease (OR, 2.79; p < 0.001).52
These findings collectively suggest that prolonged PPI use may be associated with a greater risk of adverse outcomes in IBD patients, emphasizing the need for cautious prescription of PPIs in these groups of patients. Clinicians should consider alternatives, such as H2RAs, for example, ranitidine, famotidine to manage gastrointestinal symptoms in IBD patients.
Bacterial Peritonitis
Several studies have investigated the association between PPI use and the risk of bacterial infections, particularly in cirrhotic patients. These studies have consistently highlighted the elevated risk of spontaneous bacterial peritonitis (SBP) associated with PPI use (Table 5). Cohort studies have shown that PPI use independently increased the risk of SBP in cirrhotic patients with ascites.54,57,72 Hospitalized cirrhotic patients, especially those above the age of 60 years using PPIs, have reported significantly higher incidences of bacterial infections, including SBP, compared with non-PPI users.73 Another study in cirrhosis patients revealed that PPI use is associated with an increased risk of SBP as well as hepatic encephalopathy (HE), highlighting a potential link between PPIs, gut bacteria translocation, and its associated adverse outcomes.74 A comprehensive meta-analysis also confirmed a two-fold increased risk of SBP as well as overall bacterial infections in cirrhotic patients using PPIs compared with nonusers.55 Apart from cirrhotic patients, the increased risk of PPI-associated SBP has also been reported in patients with hepatitis B virus (HBV)-related acute-on-chronic liver failure (ACLF).56 Thus, it is evident from the literature that PPI use, and not H2RA, is strongly associated with an increased risk of bacterial infections, particularly in cirrhotic patients, and warrants judicious use of PPIs in this subset of the patient population.
Fundic Gland Polyps
A continuous increase in the prevalence of fundic gland polyps (FGP) since 2000 has been linked with PPI use for at least 1 year (Table 5).61 In multiple reports, prolonged PPI use for more than 48 months has been identified as an independent predictor for the development of FGP.58 Long-term PPI use for more than 5 years showed a fourfold increase in FGP risk, likely due to parietal cell hyperplasia and protrusion resulting from acid suppression.75 Systematic reviews and meta-analyses have also confirmed an increased risk of FGPs in those using PPIs for at least 6–12 months or longer.59,60 PPI use for a prolonged duration of time increases gastrin levels with subsequent development of fundic gland polyps.76 Furthermore, increased risk of gastric hyperplastic polyps (HPP) during PPI therapy has been linked with H. pylori infection and high serum gastrin levels.77 These findings highlight the need for judicious use of AST, especially PPIs for prolonged duration of therapy.
Microscopic Colitis
Multiple studies have reported the association between PPI use and microscopic colitis, as presented in Table 5. Current literature supports that the ongoing use of PPIs is linked to an increased risk of microscopic colitis, with the highest risk observed when PPIs and NSAIDs are used concurrently. In multiple studies, PPIs have demonstrated a consistent association with microscopic colitis.63,78,79 Additionally, a broader analysis of more than 10,000 microscopic colitis patients over a decade reinforced the relationship between current PPI use and microscopic colitis.64 Higher exposure to PPIs is significantly linked to microscopic colitis.62 The evidence linking PPI use to microscopic colitis, especially when used alongside NSAIDs, highlights the importance of careful consideration when prescribing PPIs.
Renal Side Effects Associated with AST
Multiple studies have identified that patients on AST, particularly PPIs, have a significantly higher risk of developing acute interstitial nephritis (AIN), acute kidney injury (AKI), chronic kidney disease (CKD) as well as end-stage renal disease (ESRD) compared with nonusers (Table 6).80,81 Patients diagnosed with renal disease were found to be twice as likely to have been exposed to PPIs previously as compared with those without renal disease.82 It has also been observed that long-term PPI use in CKD patients significantly increases the risk of adverse renal outcomes and mortality.83
Table 6.
Key studies associating the use of AST with adverse renal outcomes
| Study | Study characteristic | Key findings |
|---|---|---|
| Blank et al.84 | Nested case-control study N = 5,72,661 patients | Current PPI users had a significantly increased risk of AIN compared with past users (OR, 5.16) |
| Wijarnpreecha et al.85 | Meta-analysis of five studies N = 5,36,902 participants | PPI use was associated with a 1.22-fold increased risk for CKD and 1.88-fold increased risk for ESRD, while no such association was found for H2RA users |
| Nochaiwong et al.80 | Systematic review and meta-analysis N = ~2.6 million patients | PPI users had a significantly higher risk of developing AIN (RR, 3.61; p < 0.001), AKI (RR, 1.44; p = 0.013), CKD (RR, 1.36; p = 0.012), and ESRD (RR, 1.42; p < 0.001) compared with nonusers |
| Wu et al.81 | Meta-analysis N = 2,484,924 participants | PPI use was associated with a significantly increased risk of AIN (RR, 3.76), AKI (RR, 1.61), CKD (RR, 1.20) and ESRD (RR, 1.88) |
| Ikuta et al.86 | Nested case-control study N = 2,19,082 new PPI users | Current use of PPI was associated with an increased risk of AKI compared with past users (OR, 4.09) Concurrent use of PPIs with NSAIDs (OR, 3.92), cephalosporins (OR, 2.57), or fluoroquinolones (OR, 3.08) was associated with a significantly increased risk of incident AKI |
| Chen et al.87 | Real-world analysis of post-marketing surveillance data N = 19,522 cases of PPI-associated AKI | PPI-associated AKI led to an 8.94% hospitalization rate and a 5.69% fatality rate |
| Liabeuf et al.83 | Cohort study N = 3,023 CKD patients | PPI use in CKD patients was associated with significantly increased risk of ESKD (HR, 1.74) and all-cause mortality (HR, 2.42) |
| Han et al.88 | Meta-analysis of 12 observational studies N = 2,492,125 participants | PPI therapy was associated with an increased risk of AKI (adjusted RR, 1.75; p < 0.001) |
| Koh et al.89 | Cohort study N = 9,860 patients from the cardiac surgery cohort | Patients exposed to PPIs before surgery had a higher incidence of AKI (44.0 vs 40.5%) and those requiring dialysis (5.8 vs 3.7%) compared with nonusers with prolonged hospitalization |
AIN, acute interstitial nephritis; AKI, acute kidney injury; CKD, chronic kidney disease; ESKD, end-stage kidney disease; ESRD, end-stage renal disease; HR, hazard ratio; RR, risk ratio
A strong positive correlation was observed among current PPI users and the risk of developing AIN with subsequent increase in hospitalization and fatality rates.84,87 Co-therapy of PPIs with NSAIDs or specific antibiotics may also further elevate the risk of AKI.86 The AKI risk was also found to be elevated in younger patients undergoing percutaneous coronary intervention and those with diabetes.90 Preoperative use of PPIs in patients undergoing cardiac surgery has also been found to be associated with a higher incidence of AKI, prolonged hospitalization, and ICU admissions.89 Additionally, in special populations, such as pediatric patients,91 elderly,92 and those with co-morbidities like rheumatoid arthritis,93 the use of PPI was consistently associated with an increased risk of kidney injury. Meta-analysis has also confirmed the association between PPI use and development of AKI.88 PPI-associated AKI cases showed adverse outcomes, such as increased hospitalizations, disability, and life-threatening situations, including mortality, when compared with PPI-associated CKD cases.94
Multiple studies have demonstrated a significant association between PPI use and an increased risk of CKD95–98 as well as ESRD.85,99,100 Exposure to PPI is strongly linked with a fourfold increased risk of AKI and a 20% increased risk of CKD.101 A comprehensive systematic review and meta-analysis of observational studies have further reinforced these findings.102,103 The risk of PPI-associated CKD becomes evident following 3 months of exposure.95 Furthermore, studies have revealed that CKD risk increases with cumulative PPI exposure, indicating a graded dose–response relationship with greater risk at higher doses (twice-daily) compared with once-daily.104 The risk of PPI-associated CKD is independent of the presence of AKI.105,106 Studies have also emphasized the association between PPI use and an elevated risk of CKD and ESRD in patients with diabetes.107,108 Importantly, studies have also shown that compared with H2RAs, PPI use was significantly associated with an increased risk of developing AKI, CKD, and ESRD.85,91,97,100,104,105,109
Current robust evidence suggests that clinicians should carefully weigh the risks and benefits before initiating the treatment with PPIs. Clinicians should also be more cautious in prescribing PPIs in patients with preexisting renal disorders. Alternative therapy with H2RAs, such as ranitidine, famotidine, etc., should be considered in such high-risk patients.
Cardiovascular Complications Associated with AST
Multiple studies have highlighted the cardiovascular risk associated with AST, especially PPIs (Table 7). A study involving individuals without a prior history of heart attack or stroke found that the current PPI use was associated with a higher risk of first-time ischemic stroke and MI, with higher risks among long-term and high-dose users.112 A cohort study found that cumulative PPI exposure for more than 5 years significantly increased the risk of cardiovascular disease and heart failure (HF).114 The association between PPI use and adverse cardiovascular outcomes is also supported by a large population-based systematic review suggesting an increase in all-cause mortality and major cardiovascular events.113 PPI-associated higher risks of coronary artery disease, MI, HF, and all-cause mortality have also been reported in patients with type 2 diabetes.115 Thus, it has been well documented that PPI use increases the risk of MI with cardiovascular mortality. On the contrary, H2RA use does not lead to adverse cardiovascular outcomes.111 All these findings collectively raise concerns about the cardiovascular risks associated with PPI use, particularly when used for long-term and at higher doses.
Table 7.
Key studies establishing the link between AST and adverse cardiovascular outcomes
| Study | Study characteristic | Key findings |
|---|---|---|
| Charlot et al.110 | Nationwide cohort study N = 56,406 adults hospitalized for MI | PPI use was associated with an increased risk of adverse cardiovascular outcomes after discharge for MI, independent of clopidogrel use |
| Shah et al.111 | Data mining studies N = 2.9 million individuals | PPI users had a 1.16-fold increased risk of MI with a two-fold increase in cardiovascular mortality On the contrary, the use of H2RAs was not associated with an increased cardiovascular risk |
| Sehested et al.112 | Nationwide registry-based analysis N = 2,14,998 patients post upper gastrointestinal endoscopy | PPIs may be associated with an increased risk of first-time ischemic stroke (HR, 1.13; p < 0.001) and MI (HR, 1.31; p < 0.001), with greater risk among long-term users and at high doses H2RA use was not significantly associated with ischemic stroke (HR, 1.02) or MI (HR, 1.15) |
| Shiraev et al.113 | A systematic review of 27 studies N = 22,427 patients in cardiovascular mortality datasets and N = 3,54,446 patients in morbidity datasets | PPI use significantly increased the risk of all-cause mortality (OR, 1.68, p < 0.001) and rate of major cardiovascular events (OR, 1.54, p = 0.01) |
| Bell et al.114 | Cohort study N = 6,538 | Individuals with a cumulative PPI exposure exceeding 5.1 years showed a 2.02-fold higher risk of CVD and 2.21-fold higher risk of HF compared with nonusers |
| Genge et al.115 | N = 19,229 adults with T2DM | PPI use was significantly associated with higher risks of CAD (HR, 1.27), MI (HR, 1.34), HF (HR, 1.35), and all-cause mortality (HR, 1.30) |
CAD, coronary artery disease; HF, heart failure; MI, myocardial infarction; T2DM, type 2 diabetes mellitus
AST Use and Its Side Effects Associated with Bone and Joints
The use of AST, particularly PPIs, has drawn significant attention due to a wide range of its associated side effects, particularly osteoporosis-related fractures, with hip fractures being the most prevalent.116–119 Also, there is a growing body of evidence suggesting that PPI use can lead to osteoporosis and disrupt bone metabolism. Multiple studies have consistently demonstrated this association, highlighting the side effects of AST (Table 8).
Table 8.
Key studies demonstrating AST use and its side effects associated with bone and joints
| Study | Study characteristic | Key findings |
|---|---|---|
| Corsonello et al.120 | Prospective observational study N = 401 older adults discharged from acute care hospitals | PPI use was associated with a decline in basic activities of daily living over a 12-month follow-up |
| Zhou et al.121 | Meta-analysis of 18 observational studies N = 2,44,109 fracture cases | PPI users had a 26% higher risk of hip fractures, 58% higher risk of spine fractures, and 33% higher risk of fractures at any other site |
| Thaler et al.122 | Cross-sectional study N = 400 female patients hospitalized after fall | PPI use was associated with an increased risk of recurrent falls (OR, 1.92, p = 0.04) and fractures (OR, 2.15, p = 0.03) |
| Poly et al.118 | Meta-analysis of 24 observational studies N => 2 million participants | PPI users reported a significantly higher risk of hip fractures compared with nonusers (RR, 1.20; p < 0.0001) |
| Park et al.123 | Nested case-control study N = 3,50,000 patients with GERD and PUD | The risk of osteoporotic fractures increased with prolonged PPI use (p < 0.001) PPI users had a higher risk of osteoporotic fracture than H2RA users (OR, 1.37) |
| Chou et al.124 | Population-based retrospective cohort study N = 3,98,885 T2DM patients | T2DM patients on long-term PPI therapy were at greater risk of developing hip fractures (HR, 1.41) Also, patients on low-dose PPIs were associated with an increased risk of fractures |
| Ursomanno et al.125 | Retrospective study N = 635 patients with dental implants, n = 1,480 implant sites | PPI users showed more (79.80% increase) crestal implant bone loss compared with nonusers |
| Bruin et al.126 | Case-cohort study | PPI users were associated with a 2.4-fold higher risk of developing prosthetic joint infection compared with nonusers, in patients undergoing THA |
| da Maia et al.127 | Systematic review and meta-analysis | Menopausal women on PPI therapy had a significantly greater risk for fractures (RR, 1.93; p < 0.0001) |
| Klifto et al.128 | Post-hoc comparative analysis N = 281 DRF patients | A cohort of PPI users had a significantly higher incidence of median nerve injuries (12 vs 3%; p = 0.025) and radial shaft fractures (5 vs 0%; p = 0.020) compared with nonusers |
DRF, distal radius fractures; GERD, gastroesophageal reflux disease; PPI, proton pump inhibitor; PUD, peptic ulcer disease; RR, risk ratio; THA, total hip arthroplasty
Both pediatric as well as adult patients on PPI therapy are at an increased risk of developing fractures.116 The risk is more prominent in elderly postmenopausal women.122,127,129 A comprehensive meta-analysis involving a large population of patients with fractures found that PPI users were at higher risk of fractures, with hip and spine the most prominent site, compared with nonusers.121,130 Importantly, this elevated fracture risk was consistent regardless of the duration of use, even when patients were on PPIs for a short term.118,121 Although PPI use may be associated with a higher risk of bone fractures, it does not appear to impact bone mineral density.131,132 Conversely, PPI use might lower trabecular bone scores, which can be reversed after PPI discontinuation.132 Prolonged exposure to PPIs in patients with fractures can also negatively impact bone healing, leading to a delay in callus formation and might also alter the biomechanics of healing bone.116 Literature also suggests that there exists a potential interaction between PPIs and bisphosphonates, which further elevates the risk of osteoporotic fractures, especially in elderly women.133 An elevated risk of hip fractures is also observed among patients with type 2 diabetes mellitus who have received PPIs for prolonged duration.124 Long-term, daily, high-dose PPIs have also been linked to a higher propensity of DRF with a higher incidence of median nerve injuries and radial shaft fractures.128
Beyond fractures, PPI use has also been associated with functional decline among older adults, potentially impacting their basic activities of daily living and quality of life.120 PPIs have also been associated with an increased risk of prosthetic joint infection following total hip arthroplasty (THA) and crestal bone loss at implant sites in patients undergoing implant therapy.125,126 The risk of overall side effects associated with bone and joints was also found to be higher in PPI users compared with H2RAs.123
Thus, based on the current literature, it is imperative for orthopedic surgeons and primary care physicians to consider these risks before prescribing PPIs in their routine clinical practice. The existing evidence necessitates a thoughtful risk-benefit assessment when considering PPIs for long-term therapy, particularly in patients who may be at high-risk of developing osteoporosis or fractures. Safer alternatives, like H2RAs (e.g., ranitidine, famotidine, etc.), might be considered in such patients.
Side Effects Associated with the Use of AST in Children
Despite the well-defined range of approved indications, the utilization of AST has increased across the pediatric age group. Overuse of AST in the pediatric population, including infants, is a growing concern which warrants careful consideration of its use in this special population. Prolonged gastric acid suppression in children has been associated with a range of side effects, including gastrointestinal and respiratory tract infections, bone fractures, allergies, and adverse renal outcomes in the long term.15 Table 9 enlists the studies highlighting several adverse outcomes associated with AST, especially PPIs in children.
Table 9.
Key evidence summary of AST-associated side effects in children
| Potential risk | Evidence summary |
|---|---|
| Dysbiosis | PPIs alter the microbiome in the oral cavity, gut, and lungs, leading to adverse outcomes such as necrotizing enterocolitis, late-onset sepsis in premature infants, CDI, asthma, obesity, and SIBO134 |
| CDI | In a population-based, nested case-control study, PPIs had significantly higher odds (OR, 21.5) of CDI in pediatric patients as compared with H2RAs (OR, 2.64)135 PPI use is an independent risk factor for severe CDI in children136 |
| Serious infections in children | A nationwide cohort study carried out on over 1.2 million children demonstrated a 34% increased risk of serious infections in children on PPI therapy137 Elevated risk of infection was observed throughout the body, including the GI tract, ear, nose, throat, lower respiratory tract, urinary tract, and nervous system137 Both bacterial and viral infections were more common in children receiving PPIs137 |
| Hospitalization in children with oropharyngeal dysphagia | In a retrospective cohort study, the use of PPIs in pediatric patients suffering from oropharyngeal dysphagia with evidence of aspiration was associated with an increased risk of hospitalization (IRR, 1.77) and longer hospital stay (IRR, 2.51)138 |
| HA-AKI | In a multicenter retrospective cohort study involving 42,232 hospitalized children, PPI use showed a significantly higher risk of HA-AKI compared with nonusers and H2RAs, with odds ratios of 1.37 and 1.24, respectively91 |
| Fracture | A retrospective cohort analysis showed that early exposure to AST is associated with an increased risk of childhood fractures, with a higher risk associated with PPIs (HR, 1.23) than H2RAs (1.04)139 A nationwide cohort study including 115,933 pairs of children demonstrated that PPI use is linked to a slightly elevated risk of fracture of varying types (HR, 1.1)140 |
| Asthma | A nationwide cohort study that included 80,870 pairs of children and adolescents showed that those on PPI therapy had a significantly higher incidence rate of asthma (HR, 1.57)141 |
AST, acid suppression therapy; CDI, Clostridium difficile infection; GI, gastrointestinal; H2RA, H2-receptor antagonist; HA-AKI, hospital-acquired acute kidney injury; HR, hazard ratio; IRR, incidence rate ratio; PPI, proton pump inhibitor; SIBO, small intestine bacterial overgrowth
There are studies reporting the alteration of the microbiome in pediatric patients due to PPI therapy, which can lead to adverse outcomes, including infections and gastrointestinal disorders. The role of probiotics in mitigating these effects is still uncertain, highlighting the need for further research.134 The risk of CDI is significantly increased with AST use in children. Studies have shown a higher risk with PPIs as compared with H2RAs. This association is evident even in infants and older children, emphasizing the need for careful consideration of AST in this population.135 Furthermore, specific risk factors, such as age over four years and prior PPI use, are associated with severe CDI in the pediatric population.136 A study conducted by Lassalle and co-workers reported that PPI use was associated with a 34% increased risk of serious infections in young children across various body sites, including the digestive tract, respiratory, and nervous system.137 Furthermore, PPI use in children has also been linked to an increased risk of asthma, especially in infants and toddlers with specific PPIs associated with varying degrees of risk. In a study evaluating the association between PPI use and risk of asthma in children, the HRs for esomeprazole were 1.64 (95% CI, 1.50–1.79), 1.49 (95% CI, 1.25–1.78) for lansoprazole, 1.43 (95% CI, 1.35–1.51) for omeprazole, and 2.33 (95% CI, 1.30–4.18) for pantoprazole.141 Several studies have also reported that PPI use increased the risk for fractures at various body sites, including upper and lower-limb fractures.140 This risk of fracture increases with early initiation and longer cumulative duration of treatment with acid suppressants, highlighting the need for careful consideration of AST use in infants.139 Gastric acid suppressants are also frequently prescribed in hospitalized children; however, in only 35% of the cases, PPIs were prescribed as per the approved indications.142 It has been observed in a multicenter retrospective cohort study that the risk of hospital-acquired acute kidney injury (HA-AKI) was found to be significantly higher in children on PPI therapy as compared with nonusers and H2RAs.91
Considering the uncertainty regarding the safety of PPIs in the pediatric population, the use of PPIs should only be restricted to the age group above 1 year suffering from acid peptic disorders.20 PPIs should only be prescribed if clearly indicated in pediatric patients to minimize the associated adverse effects and complications.
Deprescribing Proton Pump Inhibitors
Despite the emerging evidence on the side effects related to PPI use, there is limited awareness about deprescribing PPI therapy in order to prevent its inappropriate use.143 Uncertainty regarding the consequences of discontinuing medications and the fear that symptoms might recur are the common barriers to PPI deprescription.144 However, it is important to understand that the process of PPI deprescribing is challenging but can be accomplished through physicians' education, patients' preferences, recommendations, and professional society guidelines.4 Literature also suggests that inappropriate PPI use can be reduced significantly with a multimodal approach by focusing on patient and clinician education.145 A survey conducted among long-term AST users in an interventional reduction program reported that 70% of the patients were ready to participate in the deprescribing program.146 Studies comparing continuous PPI therapy vs on-demand therapy reported that the latter was well-tolerated among GERD patients, with approximately 80% expressing satisfaction with the on-demand approach.4 Overall, there is a need for a patient-centric deprescribing guideline that takes into account various factors, including disease-related, as well as psychological, social, financial, and physical determinants for patients. Such a comprehensive approach could enhance the effectiveness of deprescribing strategies by optimizing their design, implementation, and overall success.144
The Canadian evidence-based clinical practice guideline has suggested various strategies for deprescribing PPI, which include stopping PPI use either abruptly or through a tapering regimen, stepping down by discontinuing PPIs and replacing them with H2RAs like ranitidine, famotidine, etc., or on-demand use. The reduction of PPI use can be achieved through intermittent PPI use for a specific period to address reflux-related symptoms or esophageal lesions, on-demand PPI use to resolve symptoms with discontinuation until symptom recurrence, or by lowering the dose from a standard to a maintenance level.147 The process of deprescribing PPIs should ideally be gradual to prevent the rebound of acid secretion, which can occur due to prolonged acid suppression and associated hypergastrinemia. Abrupt withdrawal of PPIs may lead to symptom aggravation. Therefore, it is recommended to taper off PPIs carefully.4 Regardless of the chosen approach, close clinic follow-up is essential. It is recommended to schedule a follow-up within a month after initiating the deprescribing process to monitor the patient's symptoms.145
The AGA clinical practice update on the deprescribing of PPIs provided a set of consensus recommendations for managing PPI use in patients.148 It emphasizes the importance of regular reviews of the indications for PPI use and recommends that patients without a clear indication for chronic PPI use should be considered for deprescribing, and those on twice-daily dosing should be evaluated for step-down to once-daily dosing. However, patients with complicated GERD, Barrett's esophagus, eosinophilic esophagitis, idiopathic pulmonary fibrosis, or high risk for upper gastrointestinal bleeding may not be suitable for deprescribing. The decision to discontinue should be based solely on the lack of an appropriate indication. Patients discontinuing long-term PPI therapy should be informed about potential transient upper gastrointestinal symptoms. Both dose tapering and abrupt discontinuation can be considered during deprescribing.148
However, there is a lack of specific guidelines and simplified algorithms for PPI deprescribing, which might be a guiding tool for both general practitioners and patients in India.
PPI Deprescribing Algorithm
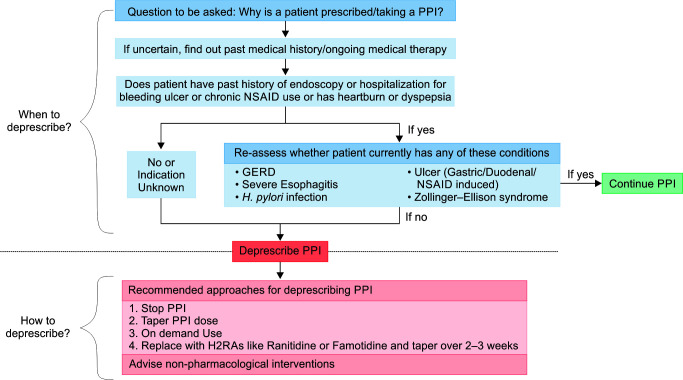
The multidisciplinary expert panel has formulated a simplified PPI deprescribing algorithm. This algorithm emphasizes the necessity of carefully evaluating the indications for continued PPI therapy or deprescribing, accounting for their appropriate and inappropriate use. The algorithm follows a stepwise approach (Fig. 2).
Fig. 2.
Proton pump inhibitors deprescribing algorithm
The initial step is to establish the rationale for the continuation of PPI therapy by asking the question as to why the patient has been prescribed PPIs. If the indication is unclear or uncertain, a thorough evaluation of the patient's past medical history and current medical therapy should be considered to understand the need for PPI therapy. For patients with a history of endoscopy, hospitalization for bleeding ulcers, chronic NSAID use, or presenting with symptoms such as heartburn or dyspepsia, a further reassessment is warranted. This assessment aims to ascertain the presence of specific conditions necessitating PPI therapy, including but not limited to GERD, severe esophagitis, H. pylori infection, gastric/duodenal/NSAID-induced ulcers, or Zollinger-Ellison syndrome. If any of these conditions are diagnosed, it is recommended to continue PPI therapy.
In cases where the patient lacks a history of endoscopy, hospitalization for bleeding ulcers, chronic NSAID use, or symptoms indicative of a specific PPI indication, and no other specific indication for PPI use is known, deprescribing is advisable. It includes a range of approaches, including PPI cessation (discontinuing PPI therapy under close monitoring), PPI dose tapering (gradual reduction of PPI dosage while monitoring symptoms), on-demand PPI use (use PPIs as and when needed rather than on a continuous basis) or replacing with H2RAs (replacing PPIs with H2RAs, such as ranitidine or famotidine). In all cases, regardless of the chosen deprescribing strategy, healthcare professionals are encouraged to implement non-pharmacological interventions to manage acid-related symptoms in patients. These may include dietary modifications, lifestyle changes, and patient education regarding symptom management and potential risks associated with long-term PPI therapy.
Consensus Statements for Rationalizing and Deprescribing Acid Suppressants
Based on the current clinical evidence and vast clinical experience, a multidisciplinary team of experts reached a consensus on the statements presented in Table 10.
Table 10.
Consensus statements agreed upon by the experts using the Delphi method
| Best practice consensus statements | Level of agreement* | Strength of expert opinion |
|---|---|---|
| Appropriate use of acid suppressants | ||
| Patients with GERD and acid-related complications (i.e., EE or peptic stricture) should receive PPI therapy for 8–12 weeks for the healing of esophagitis. The duration of therapy for symptom control should be adjusted on a case-to-case basis | 100% | Conditional |
| Strongly agree: 92% | ||
| Agree: 08% | ||
| Acid suppressants, particularly PPIs, should not be routinely co-prescribed for prophylaxis with commonly used drugs like antibiotics/iron preparations/calcium antagonists/corticosteroids/NSAIDs, etc., which are likely to cause GI disturbances | 100% | Strong |
| Strongly agree: 73% | ||
| Agree: 27% | ||
| PPIs should not be prescribed as 1st line treatment option in patients who have non-specific abdominal pain/for on-demand use when safer alternatives like H2RAs (e.g., ranitidine, famotidine) and antacids are available | 100% | Strong |
| Strongly agree: 91% | ||
| Agree: 09% | ||
| PPIs need not be prescribed in acute cases of nausea and vomiting in cases unrelated to GERD, esophagitis/PUD | 100% | Conditional |
| Strongly agree: 83% | ||
| Agree: 17% | ||
| PPIs need not be prescribed routinely in all patients taking drugs like aspirin/clopidogrel/NSAIDs/steroids/oral anticoagulants as a monotherapy, who are at low risk for GI bleeding | 100% | Conditional |
| Strongly agree: 50% | ||
| Agree: 50% | ||
| PPIs need to be routinely prescribed in patients with dual/triple antithrombotic drugs for 8–12 weeks; long-term treatment is to be considered only if patients are at high risk for GI bleeding | 100% | Conditional |
| Strongly agree: 42% | ||
| Agree: 58% | ||
| Routine prescription of PPIs in anemia patients without evidence of GI bleeding should be discouraged | 100% | Strong |
| Strongly agree: 92% | ||
| Agree: 08% | ||
| In patients on PPI therapy with persistent night-time symptoms, bedtime H2RAs like ranitidine or famotidine should be considered | 100% | Strong |
| Strongly agree: 67% | ||
| Agree: 33% | ||
| PPIs should not be used as 1st line treatment option in special populations like pregnant women experiencing heartburn and pediatric patients less than one year of age, especially when safer alternatives like H2RAs (e.g., ranitidine, famotidine) are available | 100% | Strong |
| Strongly agree: 75% | ||
| Agree: 25% | ||
| PPIs should be prescribed cautiously in patients diagnosed with conditions like IBD, microscopic colitis, spontaneous bacterial peritonitis, and fundic gland polyps | 100% | Strong |
| Strongly agree: 42% | ||
| Agree: 58% | ||
| Safety and monitoring of AST | ||
| The risk of dysbiosis, GI, and non-GI infections is higher with PPIs as compared with H2RAs in children and adults | 92% | Strong |
| Strongly agree: 33% | ||
| Agree: 58% | ||
| Neither agree or disagree: 08% | ||
| Daily long-term treatment with AST, especially PPIs, can be associated with an increased risk of vitamin (B12 and D) and mineral deficiencies (calcium, iron, and magnesium), and patients at high risk need to be monitored for nutritional deficiencies | 92% | Strong |
| Strongly agree: 42% | ||
| Agree: 50% | ||
| Neither agree or disagree: 08% | ||
| Long-term PPI use may be associated with an increased risk of adverse cardio-renal outcomes, including AKI | 100% | Strong |
| Strongly agree: 50% | ||
| Agree: 50% | ||
| Concomitant use of NSAIDs with PPIs may increase the risk of AKI | 100% | Strong |
| Strongly agree: 33% | ||
| Agree: 67% | ||
| Prolonged PPI use may be associated with an increased risk of fractures as compared with H2RAs | 100% | Strong |
| Strongly agree: 64% | ||
| Agree: 36% | ||
| Long-term PPI use (>12 weeks) in all patients with liver cirrhosis, CKD, and CVD should be avoided unless strongly indicated | 100% | Strong |
| Strongly agree: 58% | ||
| Agree: 42% | ||
| It is appropriate to monitor patients on drugs (e.g., ketoconazole, cefpodoxime, atazanavir, calcium, iron salts, etc.) with pH-dependent absorption and PPI co-medication, considering the possible drug-drug interactions | 91% | Weak |
| Strongly agree: 27% | ||
| Agree: 64% | ||
| Neither agree or disagree: 09% | ||
| Deprescribing PPIs | ||
| All patients taking PPI should undergo a regular review of the ongoing indications for use. In patients without a definitive indication, PPI should be stopped/de-prescribed | 100% | Strong |
| Strongly agree: 58% | ||
| Agree: 42% | ||
| Most patients with an indication for long-term PPI use who are currently on twice-daily dose can be considered for step down to once-daily PPI | 100% | Strong |
| Strongly agree: 50% | ||
| Agree: 50% | ||
| In patients who have completed a course of PPI treatment, resulting in the resolution of symptoms, PPI therapy should be stopped, and on-demand use of H2RAs like ranitidine/famotidine may be considered, as clinically needed | 100% | Strong |
| Strongly agree: 80% | ||
| Agree: 20% | ||
| Patients discontinuing long-term PPI therapy should be advised about transient upper GI symptoms due to rebound hyperacid secretion | 100% | Strong |
| Strongly agree: 83% | ||
| Agree: 17% |
*Level of the agreement includes strongly agree and agree votes; AST, acid suppressant therapy
Conclusion
This consensus paper developed through a collaboration of multidisciplinary experts is intended to be a valuable resource for healthcare professionals across various specialties managing patients with acid peptic disorders. It highlights the significance of evidence-based decision-making, patient-centric care, and achieving a balance between the benefits and risks associated with AST. Also, the PPI deprescribing algorithm developed by a multidisciplinary expert panel offers a systematic and evidence-based framework for healthcare professionals to assess and monitor ongoing PPI therapy. The consensus statements shall enhance the understanding and offer guidance regarding the judicious use of acid suppressants for better patient outcomes and to reduce overall healthcare costs.
Clinical Significance
This consensus paper provides clinical practice-based recommendations for optimizing the use of acid suppressants, especially PPIs in children and adults. It offers evidence-based consensus statements for primary care practitioners on the rational use of AST to improve patient outcome. Furthermore, the interdisciplinary team of experts collaboratively developed a simplified PPI deprescribing algorithm to enhance patient safety and reduce inappropriate use.
Acknowledgments
The authors acknowledge Dr Jay Savai, Dr Kapil Dev Mehta and Dr Sayali Chaudhari from JB Pharmaceuticals Ltd., Mumbai for the smooth conduct of the consensus development process. The authors also acknowledge Dr Mayuresh Garud, Scientific Director, SciPrez, Pune, for providing medical writing support.
Author Contributions
UAP and RYP conceptualized, initiated, and guided the consensus development. UAP and RYP also coordinated expert panel meetings to ensure comprehensive input. They actively engaged with stakeholders, collected feedback, and coordinated collaborative efforts.
All authors were involved in reviewing the literature specific to their respective domains and facilitated the integration of diverse perspectives. They ensured a comprehensive representation of the various viewpoints within the consensus.
The drafting of the manuscript was directed by UAP, with substantial contributions from RYP. All authors critically reviewed and provided input on the initial draft of the manuscript. UAP and RYP finalized the drafted manuscript with inputs from other authors as required. All authors read and approved the final version of the manuscript.
Footnotes
Source of support: This work was supported by JB Pharmaceuticals Ltd., Mumbai. JB Pharmaceuticals Ltd. provided an unrestricted grant for medical writing services in accordance with Good Publication Practice guidelines.
Conflict of interest: None
References
- 1.Wilks A, Panahi L, Udeani G, et al. In: Chai J (ed), editor. 2023. Review of Gastroesophageal Reflux Pharmacotherapy Management. In: Gastroesophageal Reflux Disease – A Growing Concern, IntechOpen, [DOI] [Google Scholar]
- 2.Wiggins EH, Burgess LH, Kramer J. Reducing Unnecessary Acid Suppression Use in Hospitalized Patients: A Description of Targeted Strategies Implemented Across a Large Health System. HCA Healthc J Med. 2023;4(2):125–137. doi: 10.36518/2689-0216.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simadibrata DM, Syam AF, Lee YY. A comparison of efficacy and safety of potassium-competitive acid blocker and proton pump inhibitor in gastric acid-related diseases: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2022;37(12):2217–2228. doi: 10.1111/jgh.16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mari A, Marabotto E, Ribolsi M, et al. Encouraging appropriate use of proton pump inhibitors: Existing initiatives and proposals for the future. Expert Rev Clin Pharmacol. 2023;16(10):913–923. doi: 10.1080/17512433.2023.2252327. [DOI] [PubMed] [Google Scholar]
- 5.Koggel LM, Lantinga MA, Büchner FL, et al. Predictors for inappropriate proton pump inhibitor use: Observational study in primary care. Br J Gen Pract. 2022;72:e899–906. doi: 10.3399/BJGP.2022.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Zhu X, Li R, et al. Proton pump inhibitor utilisation and potentially inappropriate prescribing analysis: Insights from a single-centred retrospective study. BMJ Open. 2020;10:e040473. doi: 10.1136/bmjopen-2020-040473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akram F, Huang Y, Lim V, et al. Proton pump inhibitors: Are we still prescribing them without valid indications? Australas Med J. 2014;7:465–470. doi: 10.4066/AMJ.2014.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh AL, Tan AG, Phan HS, et al. Indication of acid suppression therapy and predictors for the prophylactic use of protonpump inhibitors vs histamine-2 receptor antagonists in a Malaysian tertiary hospital. Pharm Pract (Granada) 2015;13:633. doi: 10.18549/PharmPract.2015.03.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savarino V, Marabotto E, Zentilin P, et al. Proton pump inhibitors: Use and misuse in the clinical setting. Expert Rev Clin Pharmacol. 2018;11:1123–1134. doi: 10.1080/17512433.2018.1531703. [DOI] [PubMed] [Google Scholar]
- 10.Scarpignato C, Gatta L, Zullo A, et al. Effective and safe proton pump inhibitor therapy in acid-related diseases – A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016;14:179, s12916-016-0718-z. doi: 10.1186/s12916-016-0718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Targownik L. Discontinuing long-term PPI therapy: Why, with whom, and how? Am J Gastroenterol. 2018;113:519–528. doi: 10.1038/ajg.2018.29. [DOI] [PubMed] [Google Scholar]
- 12.Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: Expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152:706–715. doi: 10.1053/j.gastro.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 13.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. J Am Coll Cardiol. 2016;68:1082–115. doi: 10.1016/j.jacc.2016.03.513. [DOI] [PubMed] [Google Scholar]
- 14.Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: A focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: A report of the American college of cardiology foundation task force on expert consensus documents. Circulation. 2010;122:2619–2633. doi: 10.1161/CIR.0b013e318202f701. [DOI] [PubMed] [Google Scholar]
- 15.Dipasquale V, Cicala G, Spina E, et al. A narrative review on efficacy and safety of proton pump inhibitors in children. Front Pharmacol. 2022;13:839972. doi: 10.3389/fphar.2022.839972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasman EA, Ong B, Witmer CP, et al. Proton pump inhibitors in children: the good, the bad, and the ugly. Curr Allergy Asthma Rep. 2020;20:39. doi: 10.1007/s11882-020-00926-4. [DOI] [PubMed] [Google Scholar]
- 17.De Rijdt T, Spriet I, Willems L, et al. Appropriateness of acid suppression therapy. Ann Pharmacother. 2017;51:125–134. doi: 10.1177/1060028016670414. [DOI] [PubMed] [Google Scholar]
- 18.Korayem GB, Alkanhal R, Almass R, et al. Patients, prescribers, and institutional factors associated with inappropriate use of acid suppressive therapy in medical wards: An experience of a single-center in Saudi Arabia. Int J Gen Med. 2021;14:5079–5089. doi: 10.2147/IJGM.S328914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safe M, Chan WH, Leach ST, et al. Widespread use of gastric acid inhibitors in infants: Are they needed? Are they safe? World J Gastrointest Pharmacol Ther. 2016;7(4):531–539. doi: 10.4292/wjgpt.v7.i4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Bruyne P, Ito S. Toxicity of long-term use of proton pump inhibitors in children. Arch Dis Child. 2018;103:78–82. doi: 10.1136/archdischild-2017-314026. [DOI] [PubMed] [Google Scholar]
- 21.Ito T, Jensen RT. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium. Curr Gastroenterol Rep. 2010;12:448–457. doi: 10.1007/s11894-010-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heidelbaugh JJ. Proton pump inhibitors and risk of vitamin and mineral deficiency: evidence and clinical implications. Ther Adv Drug Saf. 2013;4:125–133. doi: 10.1177/2042098613482484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kieboom BCT, Kiefte–de Jong JC, Eijgelsheim M, et al. Proton pump inhibitors and hypomagnesemia in the general population: A population-based cohort study. Am J Kidney Dis. 2015;66:775–782. doi: 10.1053/j.ajkd.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: A systematic review and meta-analysis of observational studies. Ren Fail. 2015;37:1237–1241. doi: 10.3109/0886022X.2015.1057800. [DOI] [PubMed] [Google Scholar]
- 25.Srinutta T, Chewcharat A, Takkavatakarn K, et al. Proton pump inhibitors and hypomagnesemia: A meta-analysis of observational studies. Medicine. 2019;98:e17788. doi: 10.1097/MD.0000000000017788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seah S, Tan YK, Teh K, et al. Proton-pump inhibitor use amongst patients with severe hypomagnesemia. Front Pharmacol. 2023;14:1092476. doi: 10.3389/fphar.2023.1092476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarzynski E, Puttarajappa C, Xie Y, et al. Association between proton pump inhibitor use and anemia: A retrospective cohort study. Dig Dis Sci. 2011;56:2349–2353. doi: 10.1007/s10620-011-1589-y. [DOI] [PubMed] [Google Scholar]
- 28.Tran-Duy A, Connell NJ, Vanmolkot FH, et al. Use of proton pump inhibitors and risk of iron deficiency: A population-based case-control study. J Intern Med. 2019;285:205–214. doi: 10.1111/joim.12826. [DOI] [PubMed] [Google Scholar]
- 29.Douwes RM, Gomes-Neto AW, Eisenga MF, et al. Chronic use of proton-pump inhibitors and iron status in renal transplant recipients. J Clin Med. 2019;8:1382. doi: 10.3390/jcm8091382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dharmarajan TS, Kanagala MR, Murakonda P, et al. Do acid-lowering agents affect vitamin B12 status in older adults? J Am Med Dir Assoc. 2008;9:162–167. doi: 10.1016/j.jamda.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Mumtaz H, Ghafoor B, Saghir H, et al. Association of vitamin B12 deficiency with long-term PPIs use: A cohort study. Ann Med Surg. 2022;82:104762. doi: 10.1016/j.amsu.2022.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janett S, Camozzi P, Peeters GGAM, et al. Hypomagnesemia induced by long-term treatment with proton-pump inhibitors. Gastroenterol Res Pract. 2015;2015:1–7. doi: 10.1155/2015/951768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.William JH, Danziger J. Proton-pump inhibitor-induced hypomagnesemia: Current research and proposed mechanisms. World J Nephrol. 2016;5:152–157. doi: 10.5527/wjn.v5.i2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Windsant-van den Tweel AV, Annemieke M, Derijks HJ, et al. Proton pump inhibitors and hypomagnesemia in older inpatients: An observational study. Sr Care Pharm. 2022;37:623–630. doi: 10.4140/TCP.n.2022.623. [DOI] [PubMed] [Google Scholar]
- 35.Danziger J, William JH, Scott DJ, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83:692–699. doi: 10.1038/ki.2012.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hess MW, Hoenderop JGJ, Bindels RJM, et al. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther. 2012;36:405–413. doi: 10.1111/j.1365-2036.2012.05201.x. [DOI] [PubMed] [Google Scholar]
- 37.Boxer M. Iron deficiency anemia from iron malabsorption caused by proton pump inhibitors. eJHaem. 2020;1:548–551. doi: 10.1002/jha2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swarnakari KM, Bai M, Manoharan MP, et al. The effects of proton pump inhibitors in acid hypersecretion-induced vitamin B12 deficiency: a systematic review (2022) Cureus. 2022;14:e31672. doi: 10.7759/cureus.31672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson MA, Goodrich JK, Maxan ME, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749–756. doi: 10.1136/gutjnl-2015-310861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hojo M, Asahara T, Nagahara A, et al. Gut microbiota composition before and after use of proton pump inhibitors. Dig Dis Sci. 2018;63:2940–2949. doi: 10.1007/s10620-018-5122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horvath A, Rainer F, Bashir M, et al. Biomarkers for oralization during long-term proton pump inhibitor therapy predict survival in cirrhosis. Sci Rep. 2019;9:12000. doi: 10.1038/s41598-019-48352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barletta JF, Sclar DA. Proton pump inhibitors increase the risk for hospital-acquired Clostridium difficile infection in critically ill patients. Crit Care. 2014;18:714. doi: 10.1186/s13054-014-0714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trifan A, Stanciu C, Girleanu I, et al. Proton pump inhibitors therapy and risk of Clostridium difficile infection: Systematic review and meta-analysis. World J Gastroenterol. 2017;23:6500–6515. doi: 10.3748/wjg.v23.i35.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tariq R, Singh S, Gupta A, et al. Association of gastric acid suppression with recurrent clostridium difficile infection. JAMA Intern Med. 2017;177:784–791. doi: 10.1001/jamainternmed.2017.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park YH, Seong JM, Cho S, et al. Effects of proton pump inhibitor use on risk of Clostridium difficile infection: A hospital cohort study. J Gastroenterol. 2019;54:1052–1060. doi: 10.1007/s00535-019-01598-2. [DOI] [PubMed] [Google Scholar]
- 46.Seo SI, You SC, Park CH, et al. Comparative risk of Clostridium difficile infection between proton pump inhibitors and histamine‐2 receptor antagonists: a 15‐year hospital cohort study using a common data model. J of Gastro and Hepatol. 2020;35:1325–1330. doi: 10.1111/jgh.14983. [DOI] [PubMed] [Google Scholar]
- 47.Inghammar M, Svanström H, Voldstedlund M, et al. Proton-pump inhibitor use and the risk of community-associated Clostridium difficile infection. Clin Infect Dis. 2021;72:e1084–e1089. doi: 10.1093/cid/ciaa1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D'Silva KM, Mehta R, Mitchell M, et al. Proton pump inhibitor use and risk for recurrent Clostridioides difficile infection: A systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:697–703. doi: 10.1016/j.cmi.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Lee P, Fike D, Yang H, et al. Do the types and routes of proton pump inhibitor treatments affect clostridium difficile in ICU patients? A retrospective cohort study. Expert Rev Clin Pharmacol. 2021;14:399–404. doi: 10.1080/17512433.2021.1890582. [DOI] [PubMed] [Google Scholar]
- 50.Xia B, Yang M, Nguyen LH, et al. Regular use of proton pump inhibitor and the risk of inflammatory bowel disease: pooled analysis of 3 prospective cohorts. Gastroenterology. 2021;161:1842–1852.e10. doi: 10.1053/j.gastro.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Shastri SA, Kantamneni R, Rashid M, et al. Proton pump inhibitors use and risk of inflammatory bowel diseases: A meta-analysis of observational studies. Med Pharm Rep. 2022;95:357–369. doi: 10.15386/mpr-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Onwuzo S, Boustany A, Abou Zeid HK, et al. Prevalence and risk factors associated with inflammatory bowel disease in patients using proton-pump inhibitors: a population-based study. Cureus. 2023;15:e34088. doi: 10.7759/cureus.34088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choden T, Zhang H, Sakuraba A. Influence of proton pump inhibitor use on clinical outcomes of patients with inflammatory bowel disease. Ann Med. 2023;55:2198775. doi: 10.1080/07853890.2023.2198775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Min YW, Lim KS, Min BH, et al. Proton pump inhibitor use significantly increases the risk of spontaneous bacterial peritonitis in 1965 patients with cirrhosis and ascites: A propensity score matched cohort study. Aliment Pharmacol Ther. 2014;40:695–704. doi: 10.1111/apt.12875. [DOI] [PubMed] [Google Scholar]
- 55.Xu HB, Wang HD, Li CH, et al. Proton pump inhibitor use and risk of spontaneous bacterial peritonitis in cirrhotic patients: A systematic review and meta-analysis. Genet Mol Res. 2015;14:7490–7501. doi: 10.4238/2015.July.3.25. [DOI] [PubMed] [Google Scholar]
- 56.Zhang M, Xu X, Liu W, et al. Proton pump inhibitor therapy increases the risk of spontaneous bacterial peritonitis in patients with HBV-related acute-on-chronic liver failure. Adv Ther. 2021;38:4675–4694. doi: 10.1007/s12325-021-01844-1. [DOI] [PubMed] [Google Scholar]
- 57.Dahabra L, Kreidieh M, Abureesh M, et al. Proton pump inhibitors use and increased risk of spontaneous bacterial peritonitis in cirrhotic patients: a retrospective cohort analysis. Gastroenterol Res. 2022;15:180–187. doi: 10.14740/gr1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ally MR, Veerappan GR, Maydonovitch CL, et al. Chronic proton pump inhibitor therapy associated with increased development of fundic gland polyps. Dig Dis Sci. 2009;54:2617–2622. doi: 10.1007/s10620-009-0993-z. [DOI] [PubMed] [Google Scholar]
- 59.Tran-Duy A, Spaetgens B, Hoes AW, et al. Use of proton pump inhibitors and risks of fundic gland polyps and gastric cancer: Systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1706–19.e5. doi: 10.1016/j.cgh.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 60.Martin FC, Chenevix-Trench G, Yeomans ND, et al. Systematic review with meta-analysis: fundic gland polyps and proton pump inhibitors. Aliment Pharmacol Ther. 2016;44:915–925. doi: 10.1111/apt.13800. [DOI] [PubMed] [Google Scholar]
- 61.Velazquez-Dohorn M, López-Durand CF, Candanedo-González F, et al. Análisis de casos y controles de pólipos de glándulas fúndicas e inhibidores de bomba de protones. La perspectiva de un patólogo. Rev Gastroenterol Mex. 2020;85:42–47. doi: 10.1016/j.rgmx.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 62.Keszthelyi D, Jansen SV, Schouten GA, et al. Proton pump inhibitor use is associated with an increased risk for microscopic colitis: A case-control study: Proton pump inhibitors and microscopic colitis. Aliment Pharmacol Ther. 2010;32:1124–1128. doi: 10.1111/j.1365-2036.2010.04453.x. [DOI] [PubMed] [Google Scholar]
- 63.Masclee GMC, Coloma PM, Kuipers EJ, et al. Increased risk of microscopic colitis with use of proton pump inhibitors and non-steroidal anti-inflammatory drugs. Am J Gastroenterol. 2015;110:749–759. doi: 10.1038/ajg.2015.119. [DOI] [PubMed] [Google Scholar]
- 64.Bonderup OK, Nielsen GL, Dall M, et al. Significant association between the use of different proton pump inhibitors and microscopic colitis: A nationwide Danish case‐control study. Aliment Pharmacol Ther. 2018;48:618–625. doi: 10.1111/apt.14916. [DOI] [PubMed] [Google Scholar]
- 65.Imhann F, Vich Vila A, Bonder MJ, et al. The influence of proton pump inhibitors and other commonly used medication on the gut microbiota. Gut Microbes. 2017;8:351–358. doi: 10.1080/19490976.2017.1284732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bruno G, Zaccari P, Rocco G, et al. Proton pump inhibitors and dysbiosis: Current knowledge and aspects to be clarified. World J Gastroenterol. 2019;25:2706–2719. doi: 10.3748/wjg.v25.i22.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bauer SR, O'Malley P. Withholding proton pump inhibitors to prevent recurrent Clostridium difficile: Time for a randomized trial. JAMA Int Med. 2017;177:791. doi: 10.1001/jamainternmed.2017.0233. [DOI] [PubMed] [Google Scholar]
- 68.Tawam D, Baladi M, Jungsuwadee P, et al. The positive association between proton pump inhibitors and Clostridium difficile infection. Innov Pharm. 2021;12(1) doi: 10.24926/iip.v12i1.3439. 10.24926/iip.v12i1.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azab M, Doo L, Doo DH, et al. Comparison of the hospital-acquired clostridium difficile infection risk of using proton pump inhibitors vs histamine-2 receptor antagonists for prophylaxis and treatment of stress ulcers: A systematic review and meta-analysis. Gut Liver. 2017;11:781–788. doi: 10.5009/gnl16568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dos Santos-Schaller O, Boisset S, Seigneurin A, et al. Recurrence and death after Clostridium difficile infection: Gender-dependant influence of proton pump inhibitor therapy. Springerplus. 2016;5:430. doi: 10.1186/s40064-016-2058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh N, Nugent Z, Singh H, et al. Proton pump inhibitor use before and after a diagnosis of inflammatory bowel disease. Inflamm Bowel Dis. 2023;29:1871–1878. doi: 10.1093/ibd/izad017. [DOI] [PubMed] [Google Scholar]
- 72.Miura K, Tanaka A, Yamamoto T, et al. Proton pump inhibitor use is associated with spontaneous bacterial peritonitis in patients with liver cirrhosis. Intern Med. 2014;53:1037–1042. doi: 10.2169/internalmedicine.53.2021. [DOI] [PubMed] [Google Scholar]
- 73.Elzouki AN, Neffati N, Rasoul FA, et al. Increased risk of spontaneous bacterial peritonitis in cirrhotic patients using proton pump inhibitors. GE Port J Gastroenterol. 2019;26:83–89. doi: 10.1159/000487963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dam G, Vilstrup H, Watson H, et al. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology. 2016;64:1265–1272. doi: 10.1002/hep.28737. [DOI] [PubMed] [Google Scholar]
- 75.Jalving M, Koornstra JJ, Wesseling J, et al. Increased risk of fundic gland polyps during long-term proton pump inhibitor therapy. Aliment Pharmacol Ther. 2006;24:1341–1348. doi: 10.1111/j.1365-2036.2006.03127.x. [DOI] [PubMed] [Google Scholar]
- 76.Hsu W, Wu I, Kuo C, et al. Influence of proton pump inhibitor use in gastrointestinal polyps. The Kaohsiung J of Med Scie. 2010;26:76–83. doi: 10.1016/s1607-551x(10)70011-5. [DOI] [PubMed] [Google Scholar]
- 77.Hongo M, Fujimoto K. Gastric Polyps Study Group. Incidence and risk factor of fundic gland polyp and hyperplastic polyp in long-term proton pump inhibitor therapy: A prospective study in Japan. J Gastroenterol. 2010;45:618–624. doi: 10.1007/s00535-010-0207-7. [DOI] [PubMed] [Google Scholar]
- 78.Verhaegh BPM, De Vries F, Masclee AAM, et al. High risk of drug‐induced microscopic colitis with concomitant use of NSAIDs and proton pump inhibitors. Aliment Pharmacol Ther. 2016;43:1004–1013. doi: 10.1111/apt.13583. [DOI] [PubMed] [Google Scholar]
- 79.Zylberberg HM, Kamboj AK, De Cuir N, et al. Medication use and microscopic colitis: a multicentre retrospective cohort study. Aliment Pharmacol Ther. 2021;53:1209–1215. doi: 10.1111/apt.16363. [DOI] [PubMed] [Google Scholar]
- 80.Nochaiwong S, Ruengorn C, Awiphan R, et al. The association between proton pump inhibitor use and the risk of adverse kidney outcomes: A systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33:331–342. doi: 10.1093/ndt/gfw470. [DOI] [PubMed] [Google Scholar]
- 81.Wu B, Shang W, Li Y, et al. Association between proton pump inhibitors use and kidney diseases: A meta-analysis. Int J Clin Exp Med. 2018;11:6465–6473. Available from: https://e-century.us/files/ijcem/11/7/ijcem0069786.pdf. [Google Scholar]
- 82.Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: A nested case–control study. BMC Nephrol. 2013;14:150. doi: 10.1186/1471-2369-14-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liabeuf S, Lambert O, Metzger M, et al. Adverse outcomes of proton pump inhibitors in patients with chronic kidney disease: The CKD‐REIN cohort study. Brit J Clinical Pharma. 2021;87:2967–2976. doi: 10.1111/bcp.14713. [DOI] [PubMed] [Google Scholar]
- 84.Blank ML, Parkin L, Paul C, et al. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86:837–844. doi: 10.1038/ki.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wijarnpreecha K, Thongprayoon C, Chesdachai S, et al. Associations of proton-pump inhibitors and H2 receptor antagonists with chronic kidney disease: a meta-analysis. Dig Dis Sci. 2017;62:2821–2827. doi: 10.1007/s10620-017-4725-5. [DOI] [PubMed] [Google Scholar]
- 86.Ikuta K, Nakagawa S, Momo K, et al. Association of proton pump inhibitors and concomitant drugs with risk of acute kidney injury: A nested case–control study. BMJ Open. 2021;11:e041543. doi: 10.1136/bmjopen-2020-041543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen G, Ning L, Qin Y, et al. Acute kidney injury following the use of different proton pump inhibitor regimens: A real‐world analysis of post‐marketing surveillance data. J of Gastro and Hepatol. 2021;36:156–162. doi: 10.1111/jgh.15151. [DOI] [PubMed] [Google Scholar]
- 88.Han CT, Islam MdM, Poly TN, et al. A meta-analysis of proton pump inhibitor use and the risk of acute kidney injury: Geographical differences and associated factors. J Clin Med. 2023;12:2467. doi: 10.3390/jcm12072467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koh HB, Joo YS, Kim HW, et al. Association between proton pump inhibitor exposure and acute kidney injury after cardiac surgery. Mayo Clin Proc. 2023;98:266–277. doi: 10.1016/j.mayocp.2022.07.024. [DOI] [PubMed] [Google Scholar]
- 90.Cho NJ, Choi CY, Park S, et al. Association of proton pump inhibitor use with renal outcomes in patients with coronary artery disease. Kidney Res Clin Pract. 2018;37:59–68. doi: 10.23876/j.krcp.2018.37.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Y, Xiong M, Yang M, et al. Proton pump inhibitors and the risk of hospital-acquired acute kidney injury in children. Ann Transl Med. 2020;8:1438. doi: 10.21037/atm-20-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Antoniou T, Macdonald EM, Hollands S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: A population-based cohort study. CMAJ Open. 2015;3:E166–E171. doi: 10.9778/cmajo.20140074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Svanström H, Lund M, Melbye M, et al. Use of proton pump inhibitors and the risk of acute kidney injury among patients with rheumatoid arthritis: cohort study. Drug Saf. 2018;41:817–826. doi: 10.1007/s40264-018-0663-1. [DOI] [PubMed] [Google Scholar]
- 94.Wu B, Li D, Xu T, et al. Proton pump inhibitors associated acute kidney injury and chronic kidney disease: Data mining of US FDA adverse event reporting system. Sci Rep. 2021;11:3690. doi: 10.1038/s41598-021-83099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rodríguez-Poncelas A, Barceló MA, Saez M, et al. Duration and dosing of Proton Pump Inhibitors associated with high incidence of chronic kidney disease in population-based cohort. PLoS ONE. 2018;13:e0204231. doi: 10.1371/journal.pone.0204231. Aguilera AI, editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arora P, Gupta A, Golzy M, et al. Proton pump inhibitors are associated with increased risk of development of chronic kidney disease. BMC Nephrol. 2016;17:112. doi: 10.1186/s12882-016-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang XY, He QS, Jing Z, et al. Effect of proton pump inhibitors on the risk of chronic kidney disease: A propensity score-based overlap weight analysis using the United Kingdom Biobank. Front Pharmacol. 2022;13:949699. doi: 10.3389/fphar.2022.949699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guedes JVM, Aquino JA, Castro TLB, et al. Omeprazole use and risk of chronic kidney disease evolution. PLoS ONE. 2020;15:e0229344. doi: 10.1371/journal.pone.0229344. Remuzzi G, editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peng YC, Lin CL, Yeh HZ, et al. Association between the use of proton pump inhibitors and the risk of ESRD in renal diseases: A population-based, case-control study. Medicine. 2016;95:e3363. doi: 10.1097/MD.0000000000003363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hatakeyama Y, Horino T, Matsumoto T, et al. Long-term continuous use of proton-pump inhibitors is associated with renal function decline in patients without acute kidney injury. Clin Exp Nephrol. 2021;25:1087–1092. doi: 10.1007/s10157-021-02066-z. [DOI] [PubMed] [Google Scholar]
- 101.Hart E, Dunn TE, Feuerstein S, et al. Proton pump inhibitors and risk of acute and chronic kidney disease: a retrospective cohort study. Pharmacotherapy. 2019;39:443–453. doi: 10.1002/phar.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu CC, Liao MH, Kung WM, et al. Proton pump inhibitors and risk of chronic kidney disease: evidence from observational studies. J Clin Med. 2023;12:2262. doi: 10.3390/jcm12062262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vengrus CS, Delfino VD, Bignardi PR. Proton pump inhibitors use and risk of chronic kidney disease and end-stage renal disease. Minerva Urol Nephrol. 2021;73:462–470. doi: 10.23736/S2724-6051.21.04116-3. [DOI] [PubMed] [Google Scholar]
- 104.Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238. doi: 10.1001/jamainternmed.2015.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xie Y, Bowe B, Li T, et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 2017;91:1482–1494. doi: 10.1016/j.kint.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 106.Klatte DCF, Gasparini A, Xu H, et al. Association between proton pump inhibitor use and risk of progression of chronic kidney disease. Gastroenterology. 2017;153:702–710. doi: 10.1053/j.gastro.2017.05.046. [DOI] [PubMed] [Google Scholar]
- 107.Hussain S, Singh A, Habib A, et al. Proton pump inhibitors use and risk of chronic kidney disease: Evidence-based meta-analysis of observational studies. Clin Epidemiol Glob Health. 2019;7:46–52. doi: 10.1016/j.cegh.2017.12.008. [DOI] [Google Scholar]
- 108.Yang H, Juang SY, Liao KF. Proton pump inhibitors use and risk of chronic kidney disease in diabetic patients. Diabetes Res Clin Pract. 2019;147:67–75. doi: 10.1016/j.diabres.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 109.Pannoi T, Promchai C, Apiromruck P, et al. Estimates of chronic kidney diseases associated with proton-pump inhibitors using a retrospective hospital-based cohort in Thailand. Int J Novel Res Devel. 2022;15:371–381. doi: 10.2147/IJNRD.S389238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Charlot M, Ahlehoff O, Norgaard ML, et al. Proton-pump inhibitors are associated with increased cardiovascular risk independent of clopidogrel use: A nationwide cohort study. Ann Intern Med. 2010;153:378–386. doi: 10.7326/0003-4819-153-6-201009210-00005. [DOI] [PubMed] [Google Scholar]
- 111.Shah NH, LePendu P, Bauer-Mehren A, et al. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS ONE. 2015;10:e0124653. doi: 10.1371/journal.pone.0124653. Guo Y, editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sehested TSG, Gerds TA, Fosbøl EL, et al. Long‐term use of proton pump inhibitors, dose–response relationship and associated risk of ischemic stroke and myocardial infarction. J Intern Med. 2018;283:268–281. doi: 10.1111/joim.12698. [DOI] [PubMed] [Google Scholar]
- 113.Shiraev TP, Bullen A. Proton pump inhibitors and cardiovascular events: A systematic review. Heart Lung Circ. 2018;27:443–450. doi: 10.1016/j.hlc.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 114.Bell EJ, Bielinski SJ, Sauver JL, et al. Association of proton pump inhibitors with higher risk of cardiovascular disease and heart failure. Mayo Clin Proc. 2021;96:2540–2549. doi: 10.1016/j.mayocp.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Geng T, Chen JX, Zhou YF, et al. Proton pump inhibitor use and risks of cardiovascular disease and mortality in patients with type 2 diabetes. J Clin Endocrinol Metab. 2023;108:e216–e222. doi: 10.1210/clinem/dgac750. [DOI] [PubMed] [Google Scholar]
- 116.Wagner SC. Proton pump inhibitors and bone health: What the orthopedic surgeon needs to know. JBJS Rev. 2018;6:e6. doi: 10.2106/JBJS.RVW.18.00029. [DOI] [PubMed] [Google Scholar]
- 117.Targownik LE, Lix LM, Metge CJ, et al. Use of proton pump inhibitors and risk of osteoporosis-related fractures. Can Med Assoc J. 2008;179:319–326. doi: 10.1503/cmaj.071330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Poly TN, Islam MM, Yang HC, et al. Proton pump inhibitors and risk of hip fracture: A meta-analysis of observational studies. Osteoporos Int. 2019;30:103–114. doi: 10.1007/s00198-018-4788-y. [DOI] [PubMed] [Google Scholar]
- 119.Min YW, Lee YC, Kim K, et al. Proton pump inhibitor use is associated with hip fracture development: A nationwide population-based cohort study. Korean J Intern Med. 2020;35:1084–1093. doi: 10.3904/kjim.2018.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Corsonello A, Maggio M, Fusco S, et al. Proton pump inhibitors and functional decline in older adults discharged from acute care hospitals. J Am Geriatr Soc. 2014;62:1110–1115. doi: 10.1111/jgs.12826. [DOI] [PubMed] [Google Scholar]
- 121.Zhou B, Huang Y, Li H, et al. Proton-pump inhibitors and risk of fractures: An update meta-analysis. Osteoporos Int. 2016;27:339–347. doi: 10.1007/s00198-015-3365-x. [DOI] [PubMed] [Google Scholar]
- 122.Thaler HW, Sterke CS, van der Cammen TJM. Association of proton pump inhibitor use with recurrent falls and risk of fractures in older women: A study of medication use in older fallers. J Nutr Health Aging. 2016;20:77–81. doi: 10.1007/s12603-016-0679-0. [DOI] [PubMed] [Google Scholar]
- 123.Park JH, Lee J, Yu SY, et al. Comparing proton pump inhibitors with histamin-2 receptor blockers regarding the risk of osteoporotic fractures: A nested case-control study of more than 350,000 Korean patients with GERD and peptic ulcer disease. BMC Geriatr. 2020;20:407. doi: 10.1186/s12877-020-01794-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chou YS, Jiang HJ, Chen CH, et al. Proton pump inhibitor use and risk of hip fracture in patients with type 2 diabetes. Sci Rep. 2020;10:14081. doi: 10.1038/s41598-020-70712-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ursomanno BL, Cohen RE, Levine MJ, et al. Effect of proton pump inhibitors on bone loss at dental implants. Int J Oral Maxillofac Implants. 2020;35:130–134. doi: 10.11607/jomi.7800. [DOI] [PubMed] [Google Scholar]
- 126.Bruin MM, Deijkers RLM, Bazuin R, et al. Proton-pump inhibitors are associated with increased risk of prosthetic joint infection in patients with total hip arthroplasty: A case-cohort study. Acta Orthopaedica. 2021;92:431–435. doi: 10.1080/17453674.2021.1920687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Da Maia TF, De Camargo BG, Pereira ME, et al. Increased risk of fractures and use of proton pump inhibitors in menopausal women: A systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19:13501. doi: 10.3390/ijerph192013501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Klifto KM, Ruch DS, Mithani SK, et al. Outcomes associated with proton pump inhibitors and distal radius fractures: A post-hoc comparative analysis. J Plast Reconstr Aesthet Surg. 2022;75:2650–2657. doi: 10.1016/j.bjps.2022.04.013. [DOI] [PubMed] [Google Scholar]
- 129.Lewis JR, Barre D, Zhu K, et al. Long-term proton pump inhibitor therapy and falls and fractures in elderly women: a prospective cohort study. J Bone Miner Res. 2014;29:2489–2497. doi: 10.1002/jbmr.2279. [DOI] [PubMed] [Google Scholar]
- 130.Hussain S, Siddiqui AN, Habib A, et al. Proton pump inhibitors' use and risk of hip fracture: A systematic review and meta-analysis. Rheumatol Int. 2018;38:1999–2014. doi: 10.1007/s00296-018-4142-x. [DOI] [PubMed] [Google Scholar]
- 131.Nassar Y, Richter S. Proton-pump inhibitor use and fracture risk: An updated systematic review and meta-analysis. J Bone Metab. 2018;25:141–151. doi: 10.11005/jbm.2018.25.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shin YH, Gong HS, Baek GH. Lower trabecular bone score is associated with the use of proton pump inhibitors. J Clin Densitom. 2019;22:236–242. doi: 10.1016/j.jocd.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 133.Kim JJ, Jang EJ, Park J, et al. Association between proton pump inhibitor use and risk of fracture: a population-based case-control study. PLoS ONE. 2020;15:e0235163. doi: 10.1371/journal.pone.0235163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Levy EI, Hoang DM, Vandenplas Y. The effects of proton pump inhibitors on the microbiome in young children. Acta Paediatr. 2020;109:1531–1538. doi: 10.1111/apa.15213. [DOI] [PubMed] [Google Scholar]
- 135.Freedberg DE, Lamousé-Smith ES, Lightdale JR, et al. Use of acid suppression medication is associated with risk for c. Difficile infection in infants and children: A population-based study. Clin Infect Dis. 2015;61:912–917. doi: 10.1093/cid/civ432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chang TH, Hsu WY, Yang TI, et al. Increased age and proton pump inhibitors are associated with severe Clostridium difficile infections in children. J Microbiol Immunol Infect. 2020;53:578–584. doi: 10.1016/j.jmii.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 137.Lassalle M, Zureik M, Dray-Spira R. Proton pump inhibitor use and risk of serious infections in young children. JAMA Pediatrics. 2023;177:028–1038. doi: 10.1001/jamapediatrics.2023.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Duncan DR, Mitchell PD, Larson K, et al. Association of proton pump inhibitors with hospitalization risk in children with oropharyngeal dysphagia. JAMA Otolaryngol Head Neck Surg. 2018;144:1116–1124. doi: 10.1001/jamaoto.2018.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Malchodi L, Wagner K, Susi A, et al. Early acid suppression therapy exposure and fracture in young children. Pediatrics. 2019;144:e20182625. doi: 10.1542/peds.2018-2625. [DOI] [PubMed] [Google Scholar]
- 140.Wang YH, Wintzell V, Ludvigsson JF, et al. Association between proton pump inhibitor use and risk of fracture in children. JAMA Pediatr. 2020;174:543–551. doi: 10.1001/jamapediatrics.2020.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang YH, Wintzell V, Ludvigsson JF, et al. Association between proton pump inhibitor use and risk of asthma in children. JAMA Pediatr. 2021;175:394–403. doi: 10.1001/jamapediatrics.2020.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Arnoux A, Bailhache M, Tetard C, et al. Proton pump inhibitors are still overprescribed for hospitalized children. Arch Pediatr. 2022;29:258–262. doi: 10.1016/j.arcped.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 143.Gendre P, Mocquard J, Artarit P, et al. (De)Prescribing of proton pump inhibitors: what has changed in recent years? An observational regional study from the French health insurance database. BMC Prim Care. 2022;23:341. doi: 10.1186/s12875-022-01941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Al-Aly Z, Maddukuri G, Xie Y. Proton pump inhibitors and the kidney: Implications of current evidence for clinical practice and when and how to deprescribe. Am J Kidney Dis. 2020;75:497–507. doi: 10.1053/j.ajkd.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 145.Lin D, Eke C, Cai C, et al. Decreasing overall and inappropriate proton pump inhibitor use: Perspective from a large safety-net healthcare system. Clin Gastroenterol Hepatol. 2020;18:763–766.e2. doi: 10.1016/j.cgh.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 146.Smeets HM, De Wit NJ, Delnoij DMJ, et al. Patient attitudes towards and experiences with an intervention programme to reduce chronic acid-suppressing drug intake in primary care. Eur J Gen Pract. 2009;15:219–225. doi: 10.3109/13814780903452168. [DOI] [PubMed] [Google Scholar]
- 147.Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: Evidence-based clinical practice guideline. Can Fam Physician. 2017;63:354–364. 28500192 [PMC free article] [PubMed] [Google Scholar]
- 148.Targownik LE, Fisher DA, Saini SD. AGA clinical practice update on de-prescribing of proton pump inhibitors: Expert review. Gastroenterology. 2022;162:1334–1342. doi: 10.1053/j.gastro.2021.12.247. [DOI] [PubMed] [Google Scholar]