Abstract
Objectives
To evaluate the patients with mild autonomous cortisol secretion (MACS) by means of choroidal thickness (CT) and also investigate whether CT may be a diagnostic tool in the management of MACS or not.
Methods
Twenty-seven patients with MACS and 25 age-sex-matched healthy controls were enrolled in this cross-sectional comparative study. All the participants underwent CT measurement by using Spectralis optical coherence tomography (Heidelberg Engineering, Heidelberg, Germany) with enhanced deep imaging mode at the subfoveal, 500-1000-1500 µm nasal and 500-1000-1500 µm temporal to the foveola.
Results
The groups were similar in terms of spherical equivalence, age and axial lengths. The mean CT was significantly thicker in patients with MACS than controls in all measurement quadrants (p<0.001). There was no significant correlation between CT, size of the adenoma, basal cortisol, 1mg dexamethasone suppression test, salivary cortisol, 24-hour total urine-free cortisol, ACTH and DHEAS levels. However, 2 mg dexamethasone suppression test results were found to be significantly correlated with CT in temporal 500-1000 and 1500 µm quadrants (r=0.436, p=0.023, r=0.443, p=0.021 and r=0.488, p=0.010, respectively). Five (18.5%) eyes had pachychoroid pigment epitheliopathy in the MACS group.
Conclusion
CT increases in patients with MACS and those tend to have pachychoroid pigment epitheliopathy more frequent than healthy individuals. A thicker choroid in the patients with MACS may be a novel biomarker both as a diagnostic tool for the degree of hypercortisolemia and cortisol-related comorbidity.
Keywords: Choroidal thickness, mild autonomous cortisol secretion, optical coherence tomography, possible autonomous cortisol secretion
Adrenal incidentaloma is a common endocrine disorder affecting approximately 2% of the general population.[1] Mild autonomous cortisol secretion (MACS) is the most common hormonal abnormality diagnosed in 30% to 50% of the patients with adrenal mass.[2] MACS management is still a debated topic even though the situation was first described in 1974.[3] The patients with MACS present with adrenocorticotropic hormone (ACTH) independent autonomous cortisol excess without phenotypical signs of hypercortisolism.[4]
European 2016 guideline suggested to categorize patients formerly called by multiple names including subclinical Cushing’s syndrome (CS), subclinical hypercortisolism, preclinical CS according to serum cortisol levels after overnight 1mg dexamethasone suppression test (DST). Patients without clinical signs of overt CS but serum cortisol level post 1mg DST >5 µg /dl defined by the term ‘autonomous cortisol secretion’ and patients with serum cortisol level post 1 mg DST between 1.9-5 µg/dl defined by the term ‘possible autonomous cortisol secretion’.[5]
Then, European 2023 guideline proposed the term ‘’mild autonomous cortisol secretion’’ (MACS) for patients without clinical signs of overt CS but serum post DST>1.8 µg/d L and mentioned an increased risk of potentially cortisol-related morbidity and mortalities. According to the guide, additional tests to assess the degree of cortisol secretion might be useful and management strategies should be based on the degree of cortisol secretion and relevant comorbidities.[6]
1 mg DST has the highest sensitivity for initial screening and a combination of at least two abnormal tests of the Hypothalamic pituitary axis (HPA) axis including 24-hour urinary free cortisol (UFC), ACTH levels, midnight serum cortisol levels, dehydroepiandrosterone sulfate (DHEA-S) levels, salivary cortisol levels have been advocated by most experts but there is no consensus on how to establish the biochemical diagnosis.[7]
Although the lack of symptoms and progression toward overt CS is rare, MACS may induce long-term adverse conditions related to hypercortisolemia such as hypertension, insulin resistance, type 2 diabetes mellitus (DM), obesity, metabolic syndrome and increased mortality.[8] So, how to define and manage mild chronic increase in cortisol secretion remains a great challenge.
Various studies suggest that cortisol is a risk factor for choroidal thickness (CT).[9-11] In addition, the relation between the central serous chorioretinopathy (CSC), which is a member of pachychoroid spectrum disorders, characterized by serous retinal detachments, a well-known cause of visual impairment and its association with hypercortisolemia is well-documented.[12] Nevertheless, the exact correlation between cortisol levels and choroidal thickening is controversial in recent studies.[9-11]
As mentioned in the guidelines, we should screen patients with MACS for comorbidities that may be related to cortisol. These patients are a good candidate for evaluating the effect of serum cortisol levels on choroidal thickness. Thus, in this current study we primarily evaluate the patients with MACS in means of CT. In addition, we investigate which additional test to confirm the diagnosis of MACS and the degree of cortisol level correlate with CT most. We also investigate whether CT may be a diagnostic tool in the management of MACS or not.
Methods
This observational and cross-sectional study was carried out in the Department of Ophthalmology of Prof. Dr. Cemil Tascioglu City Hospital and the Department of Endocrinology and Metabolism of Sisli Hamidiye Etfal Training and Research Hospital in Türkiye in accordance with Declaration of Helsinki. It was approved by the Sisli Etfal Hamidiye Ethics Committee (registration number/date:2006/05.06.2018). All participants gave written consent.
Ophthalmic Examination
We examined both eyes of all the subjects but only the right eyes were studied for data analysis. The patients who were diagnosed as MACS were referred to ophthalmology department. Exclusion criteria were as follows; the presence of a refractive error exceeding ±3.00 diopters spherical equivalent, a history of ocular surgery and trauma, with any neurodegenerative diseases (Alzheimer, Multiple sclerosis etc.), any retinal diseases (diabetic retinopathy, macular degeneration etc.), the history of glaucoma, keratoconus and inflammatory eye diseases.
We performed a detailed ophthalmologic examination on all participants which includes best corrected visual acuity, tonometer, anterior segment-dilated fundus examination, axial length measurement and Spectralis optical coherence tomography (SD-OCT) (Heidelberg Engineering, Heidelberg, Germany). Axial length measurements were performed with Nidek Optical Biometer. Enhanced Deep Imaging (EDI) mode was used to assess the choroidal images. The vertical distance between the outer border of retinal pigment epithelium-Bruch’s membrane complex and the choroidoscleral interface was accepted as CT. Digital calipers embedded in Spectralis OCT software was used to measure CT at the subfoveal, 500-1000-1500 µm nasal and 500-1000-1500 µm temporal to the foveola (Fig. 1). All the CT measurements were performed by two experienced operators independently who were masked to the patients’ information and the average values were considered for statistical analysis. Prior to OCT imaging, all the patients underwent blood pressure measurement and systemic arterial pressure measurements higher than systolic 130 mmHg and diastolic 80 mmHg at the time of OCT examination were excluded. Considering the diurnal variation of CT, all OCT images were obtained between 2 and 4 PM.
Figure 1.
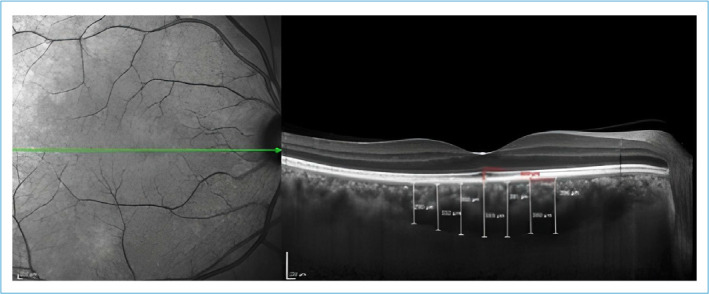
Representative image of measurement of choroidal thicknesses.
Endocrinological Evaluation
Patients group had unilateral adrenal adenoma, did not exhibit the overt signs of CS, and did not have uncontrolled DM, hypertension or malignancy. Adenomas were incidentally detected on radiological images, chemical shift analysis on MRI showed signal intensity loss in the adrenal lesions relative to reference tissue on in-phase and opposed-phase sequences. Drug history of the patients evaluated about if they were processed by cytochrome P450 3A4 for not to interfere with the results of the dynamic tests. Follow-up time, size of the adenoma, DM, and hypertension status of the patients were noted.
Control group involved otherwise healthy volunteers who did not have any cushingoid features, DM, hypertension or exogenous glucocorticoid use.
Morning plasma free cortisol (PFC) at 08 am, overnight 1mg DST, low dose 2-day 2 mg dexamethasone suppression test (LDDT), 24-hour total UFC, late-night salivary cortisol (LNCS), ACTH and DHEA-S levels were obtained. Overnight 1mg DST was the initial screening test. Mild autonomous cortisol secretion for serum cortisol level post 1mg dexamethasone >1.8 µg/dl was accepted. At least one other abnormal screening test was also positive for the patient group.
Serum cortisol levels were measured using the chemiluminescence immunoassay with commercially available kit (Beckman Coulter DXI 800 Pasadena, California, USA). For salivary cortisol reference range <2.08 µg/L for late-night between 23:30-00:30, <7.83 µg/L in the morning between 06:00-10:00, <2.43 µg/L between 16:00-20:00. Urine-free cortisol was measured with liquid chromatography-tandem mass spectrometry (LC-MS/MS) in Acibadem Lab (N reference range <45 µg /24 hour for women, <60 µg /24 hour for men). We measured DHEA-S level by the chemiluminescence method with available kit (Beckman Coulter DXI 800 Pasadena, California, USA) with age-dependent reference ranges. We used the available kit (Siemens Immulite 2000, Erlangen, Germany) to measure ACTH by the chemiluminescence method with a normal range of 0-46 ng/L.
Statistical Analysis
SPSS Statistics 21.0 (Armonk, New York: IBM Corp.) was used for statistical analyses. Normally distributed variables were given as means and standard deviations whereas non-normal variables were written as medians and percentages. After investigating whether or not the variables are normally distributed, the Student’s t-test or Mann-Whitney U test was used to compare the groups. While investigating the associations between the variables, the Spearman test or the Pearson test was preferred. A %5 type-1 error was considered to infer statistical significance.
Results
The right eyes of 27 subjects with MACS and the right eyes of 25 age-sex-matched healthy controls were evaluated in this study. All of the patients had 20/20 visual acuity. The mean age in the MACS group was 55.7±9.8 years and in controls 52.7±8.0 years. The mean axial length in MACS group was 22.6±0.3 mm and 22.7±0.3 mm in controls. The groups were similar in terms of spherical equivalence (0.3±0.7 vs 0.3±0.5; p=0.754). The clinical features of the groups are demonstrated in Table 1.
Table 1.
Clinical characteristics of the patients with MACS
| Mean | Standard Deviation | Minimum-maximum | |
|---|---|---|---|
| Age (years) | 55.7 | 9.8 | 33-68 |
| Gender (F/M) | 25/2 (92.6%) | ||
| IOP (mmHg) | 14.6 | 1.8 | 12-20 |
| Axial length (µm) | 22.6 | 0.3 | 22.2-23.4 |
| 1 mg DST (µg/dl) | 3.3 | 1.4 | 1.90-8.50 |
| 2 mg LDDT (µg/dl) | 3.6 | 1.8 | 2.0-9.7 |
| LNSC (µg/L) | 0.26 | 0.19 | 0.05-0.89 |
| UFC (µg/24h) | 90.8 | 40.9 | 42-210 |
| PFC (µg/dl) | 14.8 | 2.9 | 9.30-20 |
| ACTH (ng/L) | 7.3 | 2.3 | 4.4-11.5 |
| DHEA-S (n, %) | 4/27 (14.8%) | ||
| Follow-up time (years) | 2.8 | 2 | 1-8 |
| HbA1c (%) | 5.8 | 0.5 | 5-7 |
| Hypertension (n, %) | 15/27 (55.6%) | ||
| PreDM, DM (n, %) | 14/27 (51.8%) | ||
| Osteoporosis (n, %) | 7/27 (25.9%) | ||
| Mass size (mm) | 24.2±6.3 | 15-38 |
MACS: Mild autonomous cortisol secretion; IOP: Intraocular pressure; DST: Dexamethasone supression test; LDDT: Low dose 2-day 2 mg dexamethasone suppression test; LNSC: Late night salivary cortisol; UFC: 24-hour total urine-free cortisol; PFC: Morning plasma free cortisol.
The CT was significantly thicker in patients with MACS than controls in all measurement quadrants (p<0.001 for ETDRS areas). Table 2 summarizes the measurements of the CT (µm) in the study and control group.
Table 2.
The choroidal thickness measurements in all ETDRS areas in both groups
| Choroidal thickness | MACS group | Control group | p |
|---|---|---|---|
| Subfoveal | 371.4±114.4 | 259±41.5 | <0.001 |
| Nasal 500 µm | 344.5±111.6 | 249.4±43.3 | <0.001 |
| Nasal 1000 µm | 316.7±106.7 | 241.2±49.2 | <0.001 |
| Nasal 1500 µm | 289.9±107.5 | 215.6±50.8 | <0.001 |
| Temporal 500 µm | 344.5±107.9 | 251.1±42.4 | <0.001 |
| Temporal 1000 µm | 318.8±99.8 | 240.8±43.6 | <0.001 |
| Temporal 1500 µm | 304.9±96 | 228.4±44.8 | <0.001 |
MACS: Mild Autonomous Cortisol Secretion.
There was no significant correlation between CT, size of the adenoma, PFC, 1mg DST, LNSC, UFC, ACTH and DHEA-S levels. However, 2 mg LDDT results were found to be significantly correlated with CT in temporal 500-1000 and 1500 µm quadrants (r=0.436, p=0.023; r=0.443, p=0.021 and r=0.488, p=0.010, respectively).
None of the eyes with MACS presented with CSC, but five (18.5%) eyes had pachychoroid pigment epitheliopathy.
Discussion
In the current study, we found that the MACS patients had higher CT in all quadrans than normal subjects. Besides, they more frequently had pachychoroid pigment epitheliopathy than normal subjects. In addition, 2 mg dexamethasone suppression test results were significantly correlated with CT. To the best of our knowledge, this is the first study that evaluated CT in MACS patients.
In recent years, Karahan et al.[11] did not find any correlation between CT and PFC levels in healthy subjects. On the contrary, Karaca et al.[10] found a significant correlation between CT and ACTH and they suggested that ACTH may play an essential role in choroidal thickening more than hypercortisolemia itself. Their study includes both ACTH-independent and dependent patients. Their ACTH-dependent patients might have severe hypercortisolemia. For instance, Wang et al.[9] had adrenal gland-originating hypercortisolemic patients whose ACTH levels were suppressed and CT increased similar to our patients. They reported a positive correlation between elevated UFC rather than PFC levels and a correlation between increasing hormone levels with increase in CT. All of the patients in our study had adrenal-originated, ACTH-independent hypercortisolemia. We think that is an important point because our patients are an ideal group for the argument about if ACTH affects CT or hypercortisolemia itself.
There is no gold standard test for diagnosing MACS recommended in published guidelines.[8,13] So far 1 mg DST is known as the best initial test to diagnose MACS and additional tests are needed to confirm the diagnosis and establish the degree of cortisol excess. In our study, none of the other confirming tests but 2 mg dexamethasone suppression test results were significantly correlated with CT. Similar to our study, UFC and LNCS levels are reported frequently normal in patients with MACS.[14] In a recent retrospective study, none of those tests are found useful to discriminate between nonfunctional adrenal incidentaloma and autonomous cortisol secretion.[15] In the multicenter study designed by Araujo-Castro et al.[16] found that the diagnostic accuracy of DST for the prediction of cardiometabolic comorbidities in patients with adrenal incidentaloma was poor. Within our results, we think that 2-day LDDT positivity may better represent comorbidities and should be applied to all of the patients with MACS.
Comorbidities in MACS patients were well documented. Cardiovascular disorders, DM, insulin resistance, dyslipidemia, obesity, metabolic syndrome, non-alcoholic fatty liver, vertebral fractures, osteoporosis, impaired cognitive function, increased severity in chronic diseases and need for medications are detected in recent studies.[17-19] Considering all these comorbidities physicians must have the ability to recognize slight signs of hypercortisolism, manage on time and decide whether to observe or select adrenalectomy which improves blood pressure, glycemic and lipid control for their patients.[9, 20]
We think that it is important to show that hypercortisolemia affects CT even in subclinical conditions. So, our study may serve to emphasize the existence of an additional morbidity in patients with endogenous hypercortisolism. Also, ophthalmic examination can be another method to evaluate a patient with mild hypercortisolemia. It can be a part of the diagnostic tests to confirm hypercortisolemia and its severity. It should also not be mistaken that increase in CT, pachychoroid pigment epitheliopathy or MACS can result in visual impairment and can easily become another hypercortisolemia-related comorbidities. So, we believe that we should adjust ophthalmic examination in the patients’ workup with MACS.
Our study has limitations. Some patients had ophthalmic and endocrinological examinations on different days so we cannot rule out the fluctuation of cortisol levels. We had a relatively short duration time of adenomas (1-8 years) and a small sample size. Our findings need to be confirmed in a larger sample of patients and alternative biomarkers are needed to improve the diagnostic workup of MACS.
Conclusion
A thicker choroid in patients with MACS might be an important clue for clinicians as a diagnostic tool for hypercortisolemia status.
Footnotes
Please cite this article as ”Dogan Cakir S, Cakir A, Yener Ozturk F, Erem Basmaz S, Batman A, Saygili ES, et al. Choroidal Thickness in Mild Autonomous Cortisol Secretion. Med Bull Sisli Etfal Hosp 2024;58(2):204–209”.
Disclosures
Ethics Committee Approval
It was approved by University of Health Sciences Sisli Etfal Hamidiye Ethics Committee (registration number/date: 2006/05.06.2018).
Peer-review
Externally peer-reviewed.
Conflict of Interest
None declared.
Financial support (Funder's name)
None.
Authorship Contributions
Concept – S.D.C., A.C.; Design – S.D.C., A.C.; Supervision – F.Y.O., R.S.E., Y.A., E.C.S.; Fundings – S.E.B., A.B., E.S.S.; Materials – M.M.C., A.C., S.D.C.; Data collection &/ or processing – S.E.B., A.B., E.S.S., A.C., S.D.C., F.Y.O.; Analysis and/or interpretation – A.C., S.D.C., Y.A., F.Y.O., M.M.C.; Literature search – S.D.C., A.C.; Writing – S.D.C., A.C.; Critical review – Y.A., F.Y.O.
Use of AI for Writing Assistance
None declared.
References
- 1.Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol. 2003;149:273–85. doi: 10.1530/eje.0.1490273. [DOI] [PubMed] [Google Scholar]
- 2.Reimondo G, Castellano E, Grosso M, Priotto R, Puglisi S, Pia A, et al. Adrenal incidentalomas are tied to increased risk of diabetes: findings from a prospective study. J Clin Endocrinol Metab. 2020;105:dgz284. doi: 10.1210/clinem/dgz284. [DOI] [PubMed] [Google Scholar]
- 3.Beierwaltes WH, Sturman MF, Ryo U, Ice RD. Imaging functional nodules of the adrenal glands with 131-I-19-iodocholesterol. J Nucl Med. 1974;15:246–51. [PubMed] [Google Scholar]
- 4.Araujo-Castro M, Sampedro Núñez MA, Marazuela M. Autonomous cortisol secretion in adrenal incidentalomas. Endocrine. 2019;64:1–13. doi: 10.1007/s12020-019-01888-y. [DOI] [PubMed] [Google Scholar]
- 5.Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175:G1–34. doi: 10.1530/EJE-16-0467. [DOI] [PubMed] [Google Scholar]
- 6.Fassnacht M, Tsagarakis S, Terzolo M, Tabarin A, Sahdev A, Newell-Price J, et al. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collobration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2023;189:G1–42. doi: 10.1093/ejendo/lvad066. [DOI] [PubMed] [Google Scholar]
- 7.Nieman LK. Update on subclinical Cushing’s syndrome. Curr Opin Endocrinol Diabetes Obes. 2015;22:180–4. doi: 10.1097/MED.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 8.Sherlock M, Scarsbrook A, Abbas A, Fraser S, Limumpornpetch P, Dineen R, et al. Adrenal incidentaloma. Endocr Rev. 2020;41:775–820. doi: 10.1210/endrev/bnaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang E, Chen S, Yang H, Yang J, Li Y, Chen Y. Choroidal thickening and pachychoroid in Cushing syndrome: correlation with endogenous cortisol level. Retina. 2019;39:408–14. doi: 10.1097/IAE.0000000000001956. [DOI] [PubMed] [Google Scholar]
- 10.Karaca C, Karaca Z, Kahraman N, Sirakaya E, Oner A, Mirza GE. Is there a role of acth in increased choroidal thickness in Cushing syndrome? Retina. 2017;37:536–43. doi: 10.1097/IAE.0000000000001198. [DOI] [PubMed] [Google Scholar]
- 11.Karahan E, Zengin MO, Aydin R, Ozturk T, Kaya M, Kocak N, et al. Correlation of choroidal thickness with serum cortisol level. Clin Exp Optom. 2015;98:362–5. doi: 10.1111/cxo.12254. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho-Recchia CA, Yannuzzi LA, Negrao S, Spaide RF, Freund KB, Rodriguez-Coleman H, et al. Corticosteroids and central serous chorioretinopathy. Opthalmology. 2002;109:1834–7. doi: 10.1016/S0161-6420(02)01117-X. [DOI] [PubMed] [Google Scholar]
- 13.Park J, De Luca A, Dutton H, Malcolm JC, Doyle MA. Cardiovascular outcomes in autonomous cortisol secretion and nonfunctioning adrenal adenoma: a systematic review. J Endocr Soc. 2019;3:996–1008. doi: 10.1210/js.2019-00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bancos I, Prete A. Approach to the patient with adrenal incidentaloma. J Clin Endocrinol Metab. 2021;106:3331–53. doi: 10.1210/clinem/dgab512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueland GÅ, Grinde T, Methlie P, Kelp O, Løvås K, Husebye ES. Diagnostic testing of autonomous cortisol secretion of adrenal incidentalomas. Endocr Connect. 2020;9:963–70. doi: 10.1530/EC-20-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araujo-Castro M, Parra Ramírez P, Robles Lázaro C, García Centeno R, Gracia Gimeno P, Fernández-Ladreda MT, et al. Accuracy of the dexamethasone suppression test for the prediction of autonomous cortisol secretion-related comorbidities in adrenal incidentalomas. Hormones (Athens) 2021;20:735–44. doi: 10.1007/s42000-021-00308-z. [DOI] [PubMed] [Google Scholar]
- 17.Prete A, Subramanian A, Bancos I, Chortis V, Tsagarakis S, Lang K, et al. ENSAT EURINE-ACT Investigators Cardiometabolic disease burden and steroid excretion in benign adrenal tumors: a cross-sectional multicenter study. Ann Intern Med. 2022;175:325–34. doi: 10.7326/M21-1737. [DOI] [PubMed] [Google Scholar]
- 18.Czapla-Iskrzycka A, Świątkowska-Stodulska R, Sworczak K. Comorbidities in mild autonomous cortisol secretion–a clinical review of literature. Exp Clin Endocrinol Diabetes. 2022;130:567–76. doi: 10.1055/a-1827-4113. [DOI] [PubMed] [Google Scholar]
- 19.Liu MS, Tian ZY, Zhang Z, Yang F, Lou Y, Wang YJ, et al. Impaired cognitive function in patients with autonomous cortisol secretion in adrenal incidentalomas. J Clin Endocrinol Metab. 2023;108:633–41. doi: 10.1210/clinem/dgac603. [DOI] [PubMed] [Google Scholar]
- 20.Morelli V, Frigerio S, Aresta C, Passeri E, Pugliese F, Copetti M, et al. Adrenalectomy improves blood pressure and metabolic control in patients with possible autonomous cortisol secretion: results of a RCT. Front Endocrinol (Lausanne) 2022;13:898084. doi: 10.3389/fendo.2022.898084. [DOI] [PMC free article] [PubMed] [Google Scholar]


