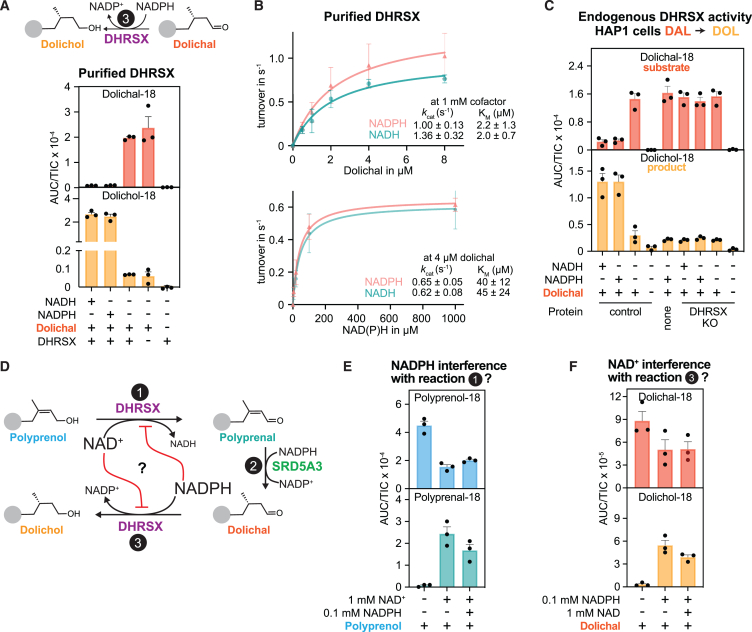
Figure 5.
DHRSX also catalyzes the final step in dolichol synthesis
(A) Formation of dolichol from dolichal was assessed after incubation of 5 μg/mL dolichal with 0.075 μmol/L recombinant DHRSX, and 1 mmol/L NAD(P)H, 2 h, 37°C. See also Figures S5A and S5B.
(B) Activity of DHRSX was determined by measuring dolichol formation after incubation of the indicated concentrations of the dolichal mixture with 1 mmol/L NADPH or NADH and 0.00375 μmol/L recombinant DHRSX for 5 min at 37°C (upper), or after an identical incubation of 4 μmol/L of the dolichal mixture with the indicated concentrations of nucleotides (lower). Presented data are turnover rates based on formation of dolichol-18 (means ± SEM; n = 3).
(C) DHRSX KO HAP1 cells lack dolichal reductase activity. Dolichol-18 and dolichal-18 were measured in reactions containing 1 mg/mL HAP1 membrane, 5 μg/mL dolichal-18, and 5 mmol/L NAD(P)H for 2 h at 37°C.
(D) Potential inhibitory interferences arising from the dual lipid and cofactor specificity of DHRSX in the revised model of dolichol synthesis. Red lines indicate potential inhibition of the opposing DHRSX activity by each reciprocal cofactor (NAD+ or NADPH). The members of each cofactor pair in larger font (NAD+ and NADPH) are those proposed to be used in vivo for DHRSX-dependent polyprenol dehydrogenase and dolichal reductase activities.
(E) The NAD+-dependent polyprenal formation from polyprenol is only mildly inhibited by NADPH concentrations found in vivo. Polyprenol-18 and polyprenal-18 were measured after a 15 min, 37°C incubation of 5 μg/mL polyprenol, 0.075 μmol/L recombinant DHRSX, and 1 mmol/L NAD+ with or without 0.1 mmol/L NADPH.
(F) The NADPH-dependent dolichol formation from dolichal is only mildly inhibited by NAD+ concentrations found in vivo. Dolichol-18 and dolichal-18 were measured after a 3 min, 37°C incubation of 5 μg/mL of dolichal, 0.075 μmol/L recombinant DHRSX, and 0.1 mmol/L NADPH with or without 1 mmol/L NAD+. Figures 5A, 5C, 5E, and 5F present TIC-normalized AUC (means ± SEM; n = 3).
See also Figure S5.