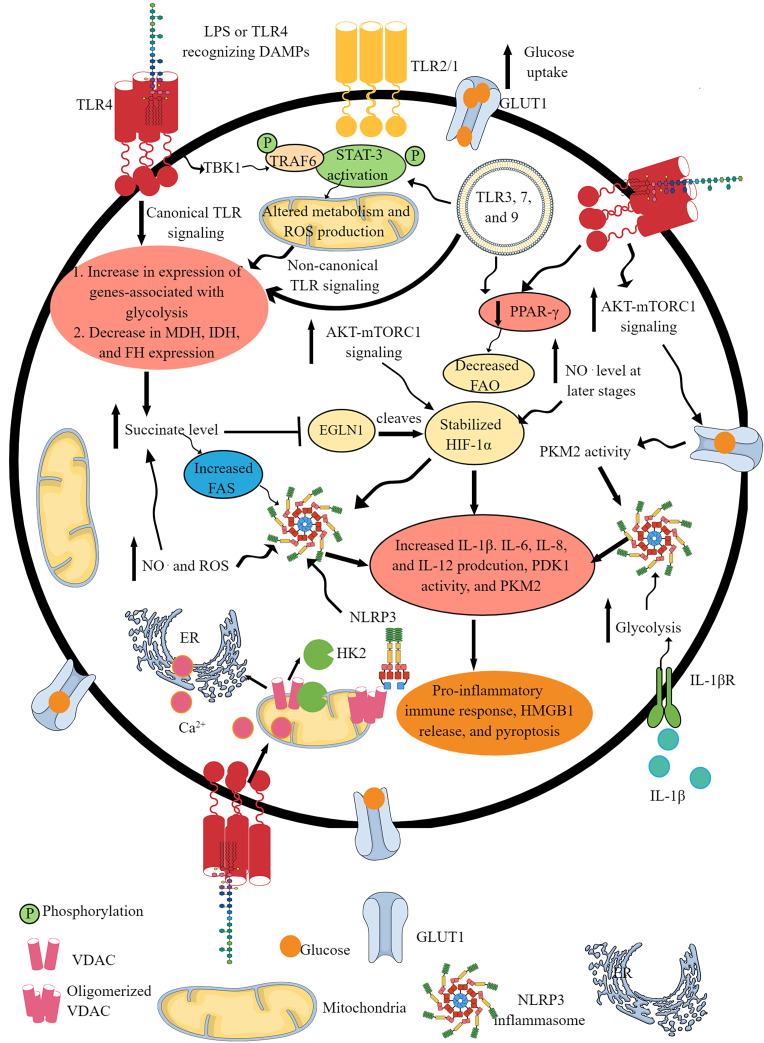
Fig. 1.
TLR and NLR signaling pathway activation-induced IR. Activation of different TLRs, such as TLR4, TLR2/1, TLR3, TLR7, and TLR9, shifts OXPHOS to glycolysis as indicated by the upregulation of glycolysis genes and downregulation of mitochondrial genes involved in OXPHOS and FAO. This IR occurs downstream of canonical and non-canonical (involves TBK1-TRAF6-STAT3 axis) TLR signaling pathways. The GLUT1 overexpression upon TLR activation further supports glycolysis by increasing glucose uptake. The TLR signaling decreases the PPAR-γ expression, which further decreases FAO to support the pro-inflammatory immune cell phenotype and function. TLR activation increases glucose uptake via increased mTOR-AKT signaling that also supports HIF-1α stabilization. The succinate accumulation upon pro-inflammatory TLR signaling activation further supports HIF-1α stabilization by inhibiting EGLN1. The NO. generation at later stages activates NLRP3 inflammasome activity and succinate accumulation. The TLR signaling-induced glycolysis, increased succinate level, HIF-1α stabilization and accumulation, PKM2, mammalian target of rapamycin complex 1, and AKT overactivity support NLRP3 inflammasome activation and IL-1β release. The HK2 dissociation from VDAC at the outer mitochondrial membrane during TLR signaling-induced glycolysis activates IP3 receptors in the ER to release Ca2+ in the cytosol – mitochondria uptake cytosolic Ca2+ molecules for VDAC oligomerization. The oligomerized VDACs aggregate with NLRP3 during its initial assembly to form the NLRP3 inflammasome complex. Furthermore, IL-1β released due to the NLRP3 inflammasome activity supports glycolysis through binding to IL-1βR. Thus, TLRs and NLRs (NLRP3) support each other’s pro-inflammatory function through IR.