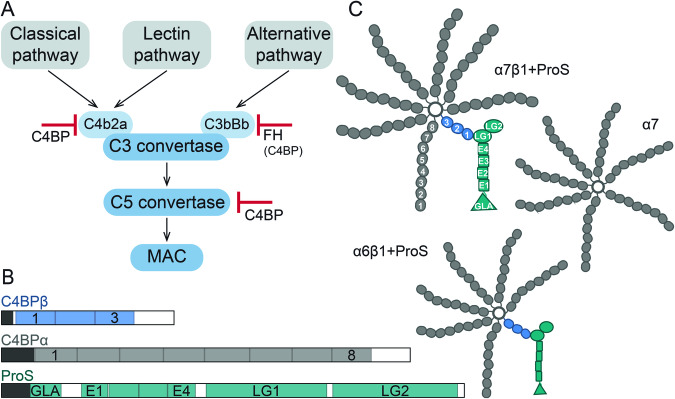
Figure 1. C4b-binding protein (C4BP) function, subunit architecture, and its proposed higher-order structures.
(A) Simplified scheme of the complement cascade activation with highlighted checkpoints controlled by C4BP. (B) Two subunits are forming the C4BP higher-order structures in human serum, namely C4BPα in gray and C4BPβ in blue. Complexing partner ProS is shown in teal. Propeptides and signal peptides are visualized in black. Complement control protein (CCP) domains of C4BPα and C4BPβ are numbered from the N- to C-terminus 1–8 and 1–3, respectively. ProS is composed of the N-terminal gamma-carboxy-glutamate domain (GLA), four EGF-like domains (E1–E4), and two Laminin G-like domains (LG1–2). (C) Cartoon representation of proposed co-occurring isoforms of human serum C4BP: α7β1+ProS, α6β1+ProS, and 7α.