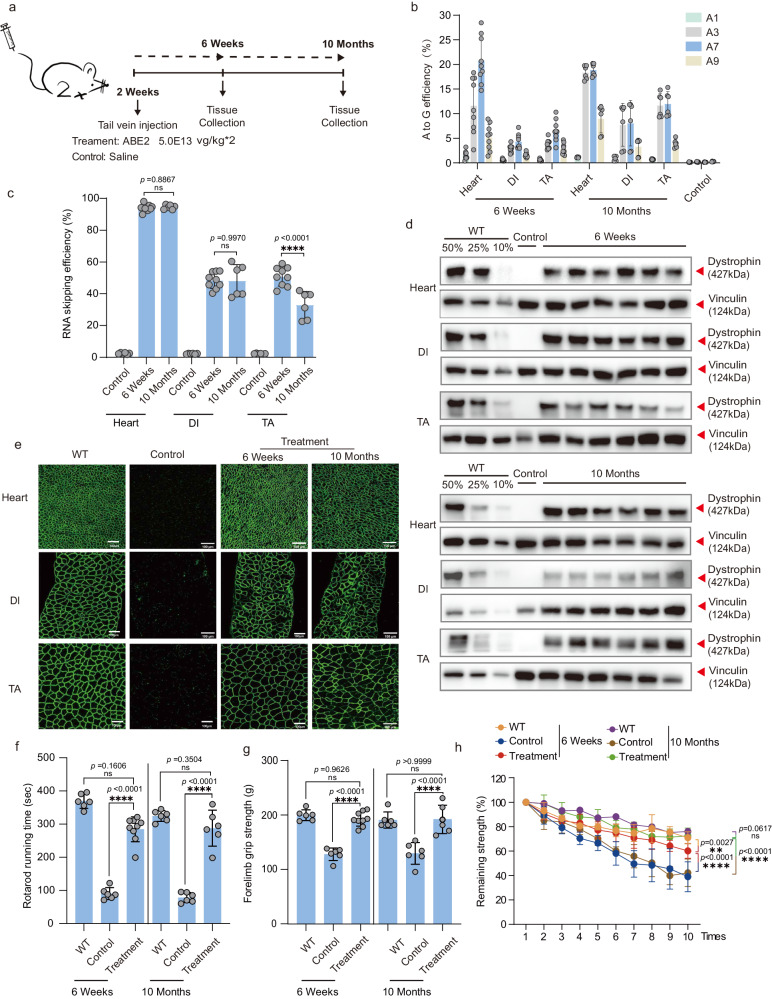
Fig. 5. Intravenous systemic delivery of ABE system efficiently rescues dystrophin expression and muscle function in 2-week-old DMD mice.
a Schematic of intravenous administration of AAV-ABE2 particles. Tissues were collected for genomic DNA, RNA, immunoblotting and immunofluorescence experiments at 6-week and 10-month after treatment. Black arrows indicate time points for tissue collection after IV injection. b Measurement by deep sequencing of splicing site mutation efficiency in TA, DI, and heart after systemic delivery. RT-PCR products from muscle of DMDΔmE5051,KIhE50/Y mice were analyzed by deep sequencing (c) to validate exon skipping efficiency. d Western blot analysis shows restoration of dystrophin expression in the tibialis anterior (TA), diaphragm (DI) and heart of DMDΔmE5051,KIhE50/Y mice 6-week and 10-month after injection. Dilutions of protein extract from WT mice were used to standardize dystrophin expression (10%, 25% and 50%). Vinculin was used as the loading control. e Immunohistochemistry for dystrophin in TA, DI, and heart of DMDΔmE5051,KIhE50/Y mice was performed 6-week and 10-month after IP injection. Dystrophin is shown in green. Scale bar, 100 μm. Rotarod rod running time (f) and Forelimb grip strength (g) was measured two days in WT, DMDΔmE5051,KIhE50/Y mice, and DMDΔmE5051,KIhE50/Y mice treated with ABE2 particles. h The remaining strength was also measured during 10 repetitions at 10-s intervals. Each dot represents an individual mouse. Data are presented as mean ± s.d (n = 6 independent biological replicates for 10-month post-treatment group and n = 9 independent biological replicates for 6-week post-treatment group). Significance is indicated by an asterisk and determined using the one-way ANOVA multiple comparison test. *P < 0.05. **P < 0.01. ****P < 0.0001, Ns represents not statistically significant. Source data are provided as a Source Data file.