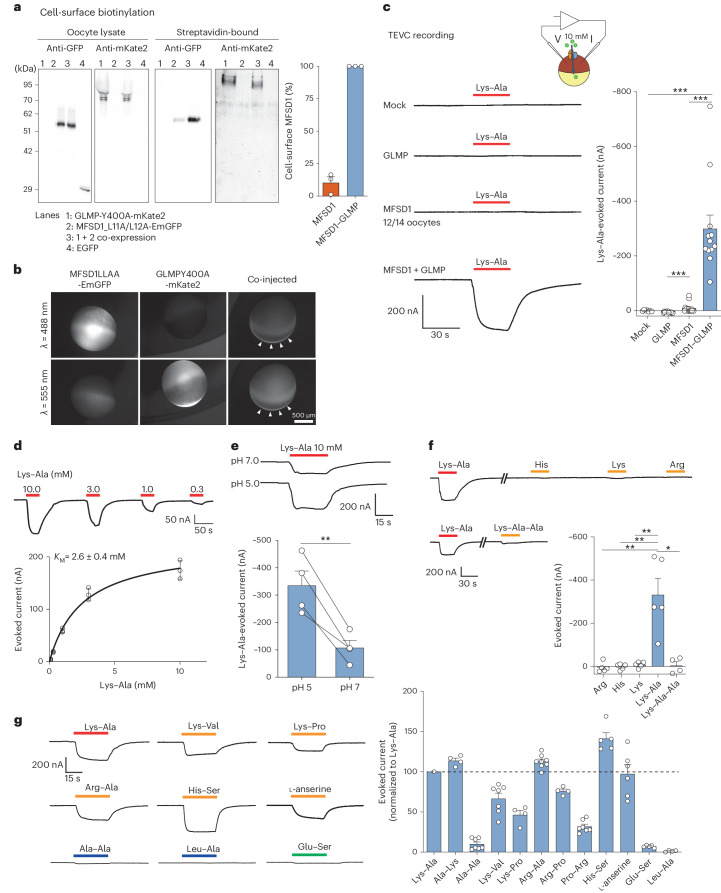
Fig. 2. Cationic dipeptides evoke an inward current in MFSD1–GLMP-expressing oocytes.
a, Surface biotinylation analysis of Xenopus oocytes expressing MFSD1L11A/L12A-EmGFP and/or GLMPY400A-mKate2. The oocytes expressing EGFP in the cytosol validated the selectivity of surface labelling in streptavidin-bound fractions. The western blots are representative of three independent experiments. b, Fluorescence micrographs of representative oocytes (n = 7 for either GLMP or MDFS1 alone and n = 25 for MFSD1 + GLMP). The arrowheads show MFSD1–GLMP colocalization at the plasma membrane. c, TEVC recording of oocytes clamped at −40 mV and perfused with 10 mM Lys–Ala at pH 5.0. The traces show representative Lys–Ala-evoked currents of 7–14 oocytes per expression condition. Only 2 out of 14 oocytes expressing only MFSD1L11A/L12A-EmGFP responded to Lys–Ala. The P values were calculated using two-sided Mann–Whitney U tests (***P ≤ 0.001). d, Dose–response relationship of the Lys–Ala current in MFSD1–GLMP oocytes. The current follows Michaelis–Menten kinetics with a KM of 2.6 ± 0.4 mM (mean ± s.e.m. of n = 3 oocytes). e, Lys–Ala was applied to each MFSD1–GLMP oocyte at pH 5.0 and pH 7.0 (mean ± s.e.m. of n = 4 oocytes). Two-tailed paired t-test, **P ≤ 0.01. f, Response of MFSD1–GLMP oocytes to cationic amino acids and to the tripeptide Lys–Ala–Ala (10 mM each) at pH 5.0. The P values were calculated using two-sided Mann–Whitney U tests, *P ≤ 0.05 and **P ≤ 0.01 (mean ± s.e.m. of n = 5 oocytes (Arg, His, Lys and Lys–Ala) and n = 4 oocytes (Lys–Ala–Ala)). g, Response of MFSD1–GLMP oocytes to diverse dipeptides compared with Lys–Ala (mean ± s.e.m. of 4–11 oocytes per substrate). The source numerical data and unprocessed blots are available in the source data.