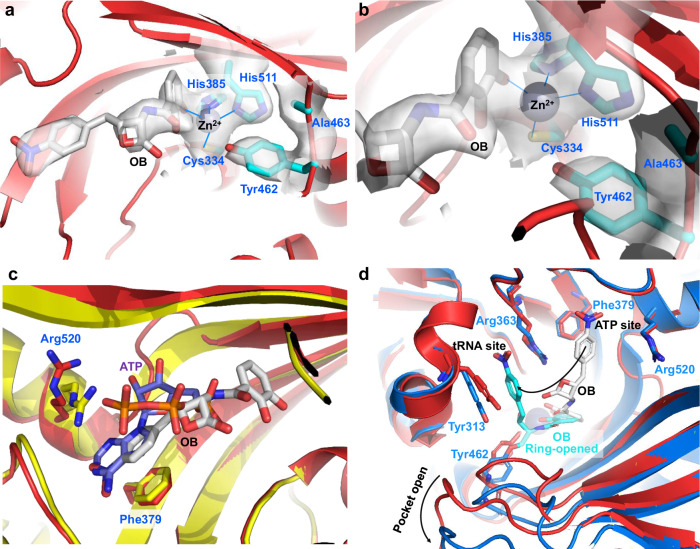
Fig. 6. OB noncovalently binds to EcThrRS_G463A without inducing a conformational change.
a, b Zoomed-in view of the catalytic pocket of EcThrRS_G463A, which was crystallized in the presence of OB. The 2Fo-Fc electron densities of OB, Cys334, His385, His511, Tyr462 and Ala463 (contoured at 1.0 σ) are shown as transparent surfaces. OB does not form an ester bond with Tyr462. c Superimposition of the EcThrRS_Y462F–ATP structure (yellow cartoons, PDB code: 8H99) with the EcThrRS_G463A–OB structure (red cartoons). The nitrophenyl group of OB (in gray) binds between Phe379 and Arg520 where the adenine group of ATP (in purple blue) stacks. The orange parts in the middle of the panel are both terminal phosphate moieties of the cocrystallized ATP. d Superimposition of the EcThrRS_WT–OB structure (marine cartoons, PDB code: 8H98) with EcThrRS_G463A–OB (red cartoons). The ring-opened OB is shown as cyan sticks. The ring-closed OB is shown as gray sticks.