Fig. 3.
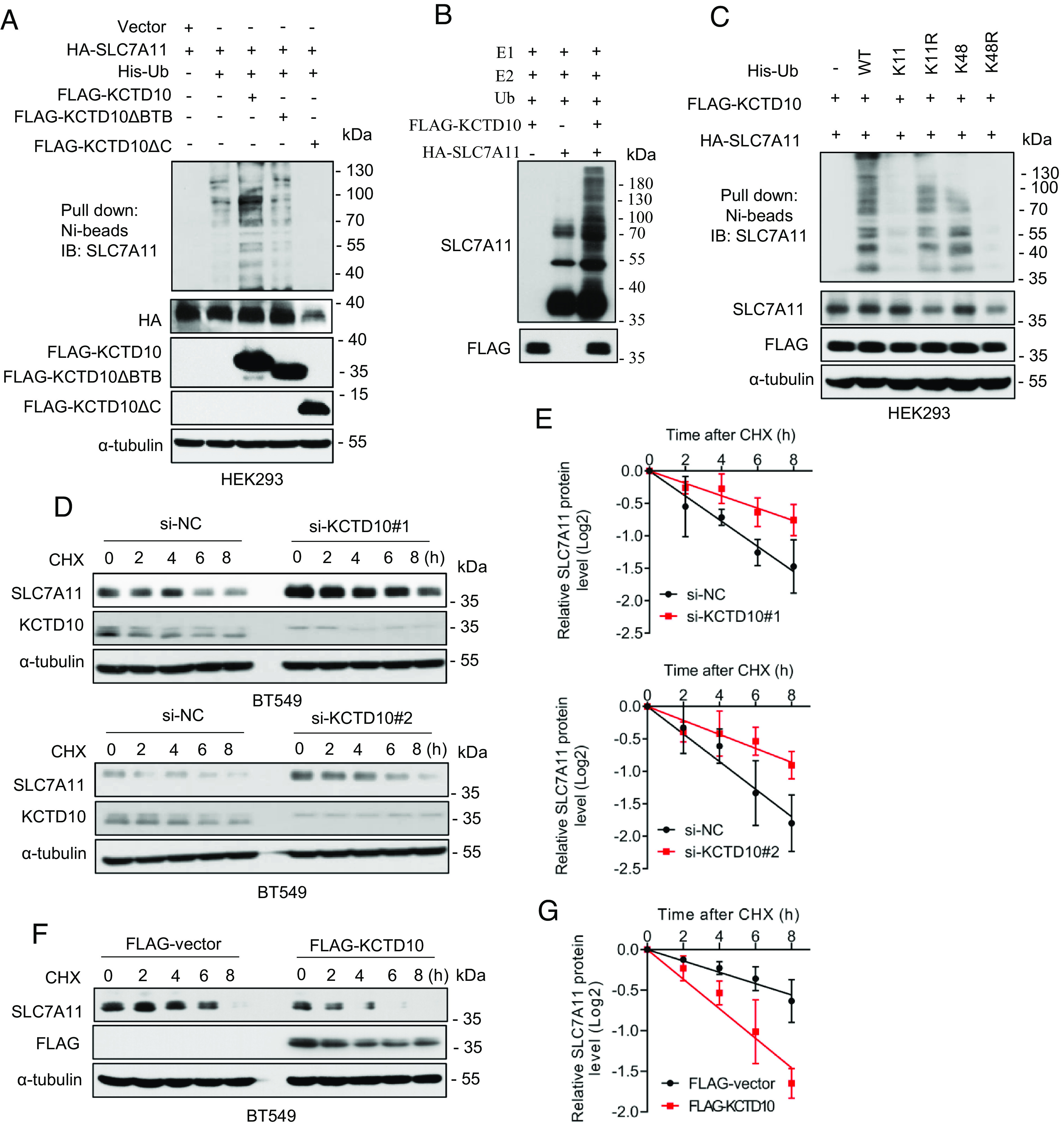
KCTD10 promotes SLC7A11 ubiquitylation and shortens SLC7A11 protein half-life. (A) HEK293 cells were transfected with indicated plasmids, followed by Ni-beads pulldown and immunoblotting for SLC7A11. (B) For in vitro ubiquitylation assay, purified HA-SLC7A11, as the substrate, was incubated with or without purified FLAG-KCTD10 in a reaction mixture containing E1 (UBE1), E2 (UbcH5a and Cdc34), ATP, and ubiquitin, followed by immunoblotting. (C) HEK293 cells were transfected with indicated plasmids, encoding wild type or mutant ubiquitins, followed by Ni-beads pulldown and immunoblotting for SLC7A11. (D and E) BT549 cells were transfected with siRNAs (si-NC or si-KCTD10s), then incubated with CHX for various time points before harvesting for immunoblotting (D), the band density of SLC7A11 was quantified and normalized to α-tubulin to draw a decay curve (mean ± SD, n = 3) (E). (F and G) BT549 cells were transfected with plasmid encoding FLAG-KCTD10, along with the vector control, then incubated with CHX for various time points before harvesting for immunoblotting (F), the band density of SLC7A11 was quantified and normalized to α-tubulin to draw a decay curve (mean ± SD, n = 3) (G).
