Fig. 4.
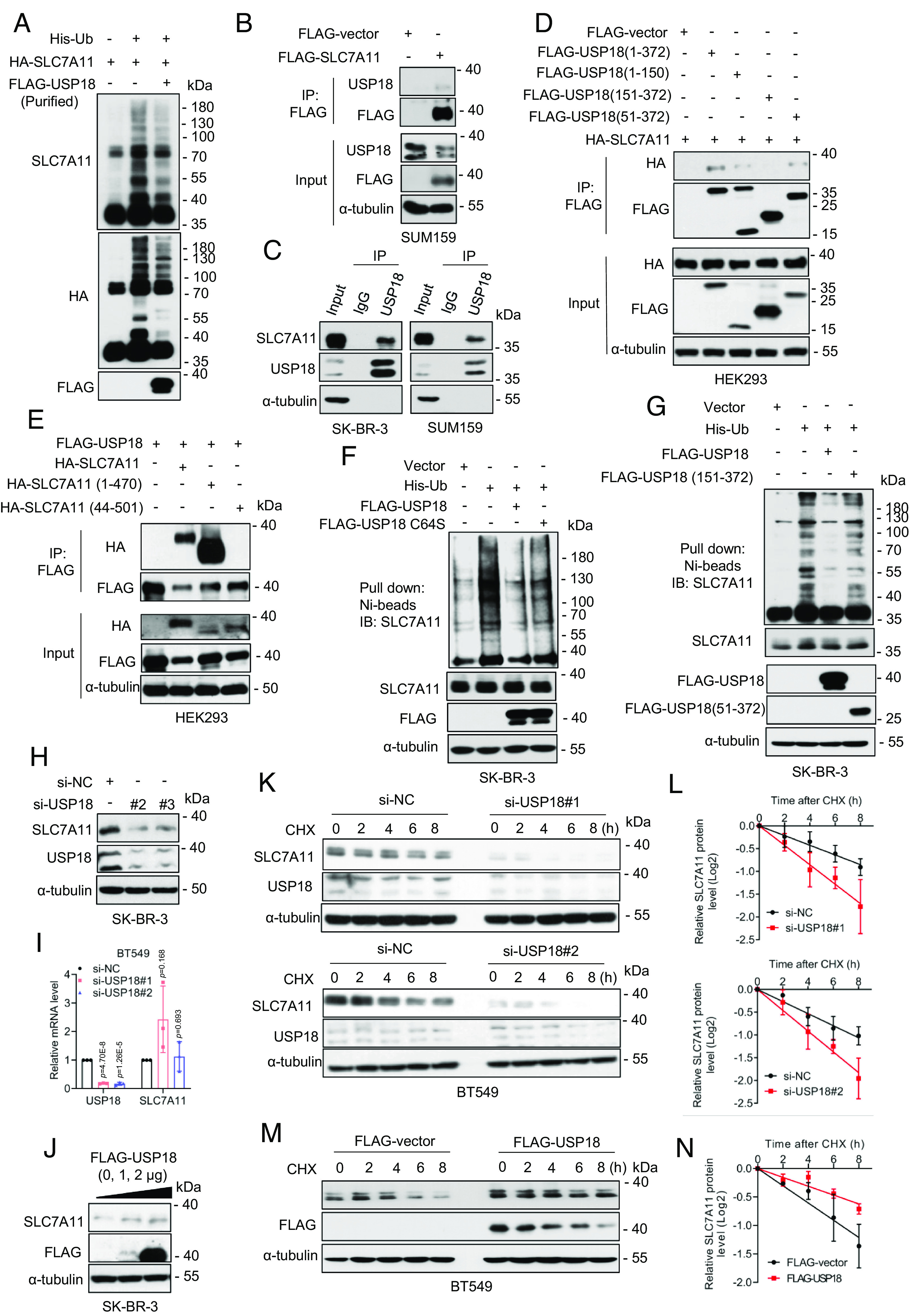
USP18 interacts with and deubiquitylates SLC7A11. (A) For in vitro deubiquitylation assay, HEK293T cells transfected with His-Ub and HA-SLC7A11. Ubiquitinated SLC7A11 was purified with IP using anti-HA Ab and was incubated without or with purified USP18, followed by immunoblotting. (B) SUM159 cells were transfected with a plasmid encoding FLAG-SLC7A11, followed by IP-IB analysis of the indicated proteins. (C) IP-IB analysis of the indicated proteins under physiological condition. (D) HEK293 cells were transfected with HA-SLC7A11 and indicated FLAG-tagged USP18 plasmids, followed by IP-IB analysis of the indicated proteins. (E) HEK293 cells were transfected with FLAG-USP18 and indicated HA-tagged SLC7A11 plasmids, followed by IP-IB analysis of the indicated proteins. (F and G) SK-BR-3 cells were transfected with indicated plasmids, followed by Ni-beads pulldown and immunoblotting for SLC7A11. (H and I) SK-BR-3 cells were transfected with siRNAs (si-NC or si-USP18s) for 48 h, followed by immunoblotting (H) and qRT-PCR analysis (mean ± SD, n = 3) (I). (J) SK-BR-3 cells were transfected with FLAG-USP18 plasmid, along with the vector control for 48 h, followed by immunoblotting. (K and L) BT549 cells were transfected with siRNAs (si-NC or si-USP18s), then incubated with CHX for various time points before harvesting for immunoblotting (K), the band density of SLC7A11 was quantified and normalized to α-tubulin to generate decay curve (mean ± SD, n = 3) (L). (M and N) BT549 cells were transfected with FLAG-USP18, along with the vector control, then incubated with CHX for various time points before harvesting for immunoblotting (M), the band density of SLC7A11 was quantified and normalized to α-tubulin to generate decay curve (mean ± SD, n = 3) (N).
