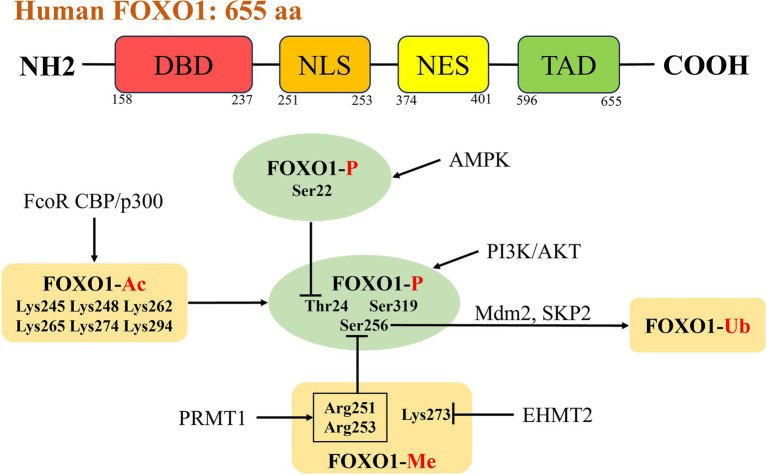
Figure 1.
Structure and activity regulation of FOXO1. The human transcription factor FOXO1 consists of 655 amino acid residues, including four functional domains: the NH2-terminal DBD (residues 158–237), the NES (residues 374–401), the NLS (residues 251–253), and the TAD (residues 596–655). There are interactions between different post-translational modifications, and PI3K/AKT signaling pathway is the most typical phosphorylation pathway for FOXO1 (phosphorylation sites Thr24, Ser256, Ser319). Acetylation of FOXO1 enhances AKT-mediated phosphorylation. Methylation of Arg251 and Arg253 inhibits phosphorylation of Ser256. Ubiquitin ligases SKP2 and Mdm2 participate in the ubiquitination and degradation of FOXO1 by binding to phosphorylated Ser256. The phosphorylation of Ser22 mediated by AMPK can interfere with the phosphorylation of Thr24 mediated by AKT. FOXO1, forkhead box O 1; DBD, DNA-binding domain; NES, nuclear export sequence; NLS, nuclear localization signal; TAD, transactivation domain; PI3K, phosphatidylinositol 3-kinase; AKT, protein Kinase B; SKP2, S-phase kinase-associated protein 2; Mdm2, murine double minute 2; AMPK, AMP-activated protein kinase.